Status and Trends of Land Degradation and Restoration and Associated Changes in Biodiversity and Ecosystem Functions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Missing Pathways to 1.5°C the Role of the Land Sector in Ambitious Climate Action
Missing Pathways to 1.5°C The role of the land sector in ambitious climate action Climate ambition that safeguards land rights, biodiversity and food sovereignty CLARA Climate Land Ambition and Rights Alliance Missing Pathways to 1.5°C The role of the land sector in ambitious climate action Lead authors: Kate Dooley, Doreen Stabinsky. Contributing authors: Kelly Stone, Shefali Sharma, Teresa Anderson, Doug Gurian-Sherman, Peter Riggs. Editor: Mark Olden Design: Andy Omel Graphics: Ethan Cornell Cover photo: Rhett A. Butler / Mongabay Acknowledgements: Hanna Aho, Mary Booth, Dominick DellaSala, Alain Frechette, Lili Fuhr, Chloe Ginsburg, Jason Hill, Claire Hoolohan, Stephanie Keene, Cyril Kormos, Sara Lickel, Stephen Leonard, Brendan Mackey, Christopher Martius, Bernard Mercer, Hannah Mowat, Anne-Laure Sable, Linda Schneider, Christoph Thiess, Karin Ulmer, Virginia Young, Linde Zuidema. Citation: Dooley, K et al. (2018) Missing Pathways to 1.5°C: The role of the land sector in ambitious climate action. Climate Land Ambition and Rights Alliance. Available from: climatelandambitionrightsalliance.org/report Contact CLARA Media: Don Lehr (CLARA) / [email protected] / +1 917 304 4058 Mark Olden (Fern) / [email protected] / +44 7973884718 Sophia Har (ActionAid) / [email protected] / +1 651 815 1818 Network Coordination: Peter Riggs (Pivot Point) / [email protected] / +1 360 426 0959 Twitter: @CLARA_Alliance Table of Contents Acronyms � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � -

Effective Population Size and Genetic Conservation Criteria for Bull Trout
North American Journal of Fisheries Management 21:756±764, 2001 q Copyright by the American Fisheries Society 2001 Effective Population Size and Genetic Conservation Criteria for Bull Trout B. E. RIEMAN* U.S. Department of Agriculture Forest Service, Rocky Mountain Research Station, 316 East Myrtle, Boise, Idaho 83702, USA F. W. A LLENDORF Division of Biological Sciences, University of Montana, Missoula, Montana 59812, USA Abstract.ÐEffective population size (Ne) is an important concept in the management of threatened species like bull trout Salvelinus con¯uentus. General guidelines suggest that effective population sizes of 50 or 500 are essential to minimize inbreeding effects or maintain adaptive genetic variation, respectively. Although Ne strongly depends on census population size, it also depends on demographic and life history characteristics that complicate any estimates. This is an especially dif®cult problem for species like bull trout, which have overlapping generations; biologists may monitor annual population number but lack more detailed information on demographic population structure or life history. We used a generalized, age-structured simulation model to relate Ne to adult numbers under a range of life histories and other conditions characteristic of bull trout populations. Effective population size varied strongly with the effects of the demographic and environmental variation included in our simulations. Our most realistic estimates of Ne were between about 0.5 and 1.0 times the mean number of adults spawning annually. We conclude that cautious long-term management goals for bull trout populations should include an average of at least 1,000 adults spawning each year. Where local populations are too small, managers should seek to conserve a collection of interconnected populations that is at least large enough in total to meet this minimum. -

Chapter 1 IPCC SRCCL
Second Order Draft Chapter 1 IPCC SRCCL 1 Chapter 1: Framing and Context 2 3 Coordinating Lead Authors: Almut Arneth (Germany) and Fatima Denton (Gambia) 4 Lead Authors: Fahmuddin Agus (Indonesia), Aziz Elbehri (Morocco), Karheinz Erb (Italy), Balgis Osman 5 Elasha (Cote d’Ivoire), Mohammad Rahimi (Iran), Mark Rounsevell (United Kingdom), Adrian Spence 6 (Jamaica) and Riccardo Valentini (Italy) 7 Contributing Authors: Peter Alexander (United Kingdom), Yuping Bai (China), Ana Bastos (Portugal), 8 Niels Debonne (The Netherlands), Thomas Hertel (United States of America), Rafaela Hillerbrand 9 (Germany), Baldur Janz (Germany), Ilva Longva (United Kingdom), Patrick Meyfroidt (Belgium), Michael 10 O'Sullivan (United Kingdom) 11 Review Editors: Edvin Aldrian (Indonesia), Bruce McCarl (United States of America), Maria Jose Sanz 12 Sanchez (Spain) 13 Chapter Scientist: Yuping Bai (China), Baldur Janz (Germany) 14 Date of Draft: 16/11/2018 15 Do Not Cite, Quote or Distribute 1-1 Total pages: 87 Second Order Draft Chapter 1 IPCC SRCCL 1 Table of Contents 2 3 Chapter 1: Framing and Context .......................................................................................................... 1-1 4 Executive summary .................................................................................................................... 1-3 5 Introduction and scope of the report .......................................................................................... 1-5 6 Objectives and scope of the assessment ............................................................................ -

Ecocide: the Missing Crime Against Peace'
35 690 Initiative paper from Representative Van Raan: 'Ecocide: The missing crime against peace' No. 2 INITIATIVE PAPER 'The rules of our world are laws, and they can be changed. Laws can restrict, or they can enable. What matters is what they serve. Many of the laws in our world serve property - they are based on ownership. But imagine a law that has a higher moral authority… a law that puts people and planet first. Imagine a law that starts from first do no harm, that stops this dangerous game and takes us to a place of safety….' Polly Higgins, 2015 'We need to change the rules.' Greta Thunberg, 2019 Table of contents Summary 1 1. Introduction 3 2. The ineffectiveness of current legislation 7 3. The legal framework for ecocide law 14 4. Case study: West Papua 20 5. Conclusion 25 6. Financial section 26 7. Decision points 26 Appendix: The institutional history of ecocide 29 Summary Despite all our efforts, the future of our natural environments, habitats, and ecosystems does not look promising. Human activity has ensured that climate change continues to persist. Legal instruments are available to combat this unprecedented damage to the natural living environment, but these instruments have proven inadequate. With this paper, the initiator intends to set forth an innovative new legal concept. This paper is a study into the possibilities of turning this unprecedented destruction of our natural environment into a criminal offence. In this regard, we will use the term ecocide, defined as the extensive damage to or destruction of ecosystems through human activity. -

Desertification and Agriculture
BRIEFING Desertification and agriculture SUMMARY Desertification is a land degradation process that occurs in drylands. It affects the land's capacity to supply ecosystem services, such as producing food or hosting biodiversity, to mention the most well-known ones. Its drivers are related to both human activity and the climate, and depend on the specific context. More than 1 billion people in some 100 countries face some level of risk related to the effects of desertification. Climate change can further increase the risk of desertification for those regions of the world that may change into drylands for climatic reasons. Desertification is reversible, but that requires proper indicators to send out alerts about the potential risk of desertification while there is still time and scope for remedial action. However, issues related to the availability and comparability of data across various regions of the world pose big challenges when it comes to measuring and monitoring desertification processes. The United Nations Convention to Combat Desertification and the UN sustainable development goals provide a global framework for assessing desertification. The 2018 World Atlas of Desertification introduced the concept of 'convergence of evidence' to identify areas where multiple pressures cause land change processes relevant to land degradation, of which desertification is a striking example. Desertification involves many environmental and socio-economic aspects. It has many causes and triggers many consequences. A major cause is unsustainable agriculture, a major consequence is the threat to food production. To fully comprehend this two-way relationship requires to understand how agriculture affects land quality, what risks land degradation poses for agricultural production and to what extent a change in agricultural practices can reverse the trend. -

Maturation at a Young Age and Small Size of European Smelt (Osmerus
Arula et al. Helgol Mar Res (2017) 71:7 DOI 10.1186/s10152-017-0487-x Helgoland Marine Research ORIGINAL ARTICLE Open Access Maturation at a young age and small size of European smelt (Osmerus eperlanus): A consequence of population overexploitation or climate change? Timo Arula*, Heli Shpilev, Tiit Raid, Markus Vetemaa and Anu Albert Abstract Age of fsh at maturation depends on the species and environmental factors but, in general, investment in growth is prioritized until the frst sexual maturity, after which a considerable and increasing proportion of resources are used for reproduction. The present study summarizes for the frst the key elements of the maturation of European smelt (Osmerus eperlanus) young of the year (YoY) in the North-eastern Gulf of Riga (the Baltic Sea). Prior to the changes in climatic conditions and collapse of smelt fshery in the 1990s in the Gulf of Riga, smelt attained sexual maturity at the age of 3–4 years. We found a substantial share (22%) of YoY smelt with maturing gonads after the collapse of the smelt fsheries. Maturing individuals had a signifcantly higher weight, length and condition factor than immature YOY, indicating the importance of individual growth rates in the maturation process. The proportion of maturing YoY individuals increased with fsh size. We discuss the factors behind prioritizing reproduction overgrowth in early life and its implications for the smelt population dynamics. Keywords: Osmerus eperlanus, Early maturation, Young of the year (0 ), Commercial fsheries + Background and younger ages [5–8]. Such shifts time of maturation Age of fsh at maturation depends on the species and might have drastic consequences for fsh population environmental factors but, in general, investment in dynamics, as the share of early maturing individuals will growth is prioritized until the frst sexual maturity, increase in population [9]. -
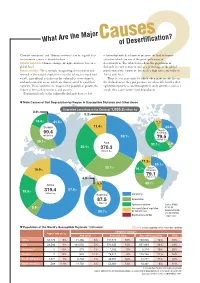
What Are the Major Causes of Desertification?
What Are the Major Causesof Desertification? ‘Climatic variations’ and ‘Human activities’ can be regarded as relationship with development pressure on land by human the two main causes of desertification. activities which are one of the principal causes of Climatic variations: Climate change, drought, moisture loss on a desertification. The table below shows the population in global level drylands by each continent and as a percentage of the global Human activities: These include overgrazing, deforestation and population of the continent. It reveals a high ratio especially in removal of the natural vegetation cover(by taking too much fuel Africa and Asia. wood), agricultural activities in the vulnerable ecosystems of There is a vicious circle by which when many people live in arid and semi-arid areas, which are thus strained beyond their the dryland areas, they put pressure on vulnerable land by their capacity. These activities are triggered by population growth, the agricultural practices and through their daily activities, and as a impact of the market economy, and poverty. result, they cause further land degradation. Population levels of the vulnerable drylands have a close 2 ▼ Main Causes of Soil Degradation by Region in Susceptible Drylands and Other Areas Degraded Land Area in the Dryland: 1,035.2 million ha 0.9% 0.3% 18.4% 41.5% 7.7 % Europe 11.4% 34.8% North 99.4 America million ha 32.1% 79.5 million ha 39.1% Asia 52.1% 5.4 26.1% 370.3 % million ha 11.5% 33.1% 30.1% South 16.9% 14.7% America 79.1 million ha 4.8% 5.5 40.7% Africa -

Fast Retailing Policy on Wood-Derived Products and Forest
Fast Retailing Responsible Product Policy: Wood-based Products and Forest Materials Ancient and endangered forests regulate our planet – providing clean air, fresh water, a stable climate and biodiversity. Fast Retailing Co., Ltd. and our brands including UNIQLO, Theory, GU, PLST, Helmut Lang, Comptoir des Cotonniers, Princess tam.tam and J Brand are committed to protecting the world’s ancient and endangered forests including efforts toward zero deforestation through our approach to procurement of wood- based fabrics, materials derived from forests, and/or manmade cellulosic fabrics. Conservation of Ancient and Endangered Forests and Ecosystems While it is commonly known that paper and wood come from forests, it is a little known fact that trees are being made into clothing. Fabrics originating from forest sources are almost exclusively referring to viscose (also known as rayon), and other fabrics are also covered in this “man-made cellulosic fabric” family. Fast Retailing Co., Ltd. is committed to undertaking reasonable efforts in the following: 1. Assess and map our existing use of forest materials and eliminate sourcing identified as coming from endangered species habitat and ancient and endangered forests. 2. Work to eliminate sourcing from companies that are logging forests illegally or tree plantations established after 1994, from areas being logged in contravention of indigenous and local peoples’ rights, and/or from other suppliers identified by Fast Retailing as controversial. 3. Should we learn that any of our forest materials are being sourced from ancient and endangered forests, endangered species habitat or through illegal logging, we will investigate our supply chain, engage our suppliers to change practices, and/or re-evaluate our relationship with them. -

An Axiomatic Foundation of the Ecological Footprint
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Kuhn, Thomas; Pestow, Radomir; Zenker, Anja Working Paper An Axiomatic Foundation of the Ecological Footprint Chemnitz Economic Papers, No. 025 Provided in Cooperation with: Chemnitz University of Technology, Faculty of Economics and Business Administration Suggested Citation: Kuhn, Thomas; Pestow, Radomir; Zenker, Anja (2018) : An Axiomatic Foundation of the Ecological Footprint, Chemnitz Economic Papers, No. 025, Chemnitz University of Technology, Faculty of Economics and Business Administration, Chemnitz This Version is available at: http://hdl.handle.net/10419/190431 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. may exercise further usage rights as specified in the indicated licence. www.econstor.eu Faculty of Economics and Business Administration An Axiomatic Foundation of the Ecological Footprint Thomas Kuhn Radomir Pestow Anja Zenker Chemnitz Economic Papers, No. -

Cropland Restoration As an Essential Component to the Forest Landscape Restoration Approach—Global Effects of Wide-Scale Adoption
IFPRI Discussion Paper 01682 October 2017 Cropland Restoration as an Essential Component to the Forest Landscape Restoration Approach—Global Effects of Wide-Scale Adoption Alessandro De Pinto, Richard Robertson, Salome Begeladze, Chetan Kumar, Ho-Young Kwon, Timothy Thomas, Nicola Cenacchi, and Jawoo Koo Environment and Production Technology Division INTERNATIONAL FOOD POLICY RESEARCH INSTITUTE The International Food Policy Research Institute (IFPRI), established in 1975, provides evidence-based policy solutions to sustainably end hunger and malnutrition and reduce poverty. The Institute conducts research, communicates results, optimizes partnerships, and builds capacity to ensure sustainable food production, promote healthy food systems, improve markets and trade, transform agriculture, build resilience, and strengthen institutions and governance. Gender is considered in all of the Institute’s work. IFPRI collaborates with partners around the world, including development implementers, public institutions, the private sector, and farmers’ organizations, to ensure that local, national, regional, and global food policies are based on evidence. AUTHORS Alessandro De Pinto ([email protected]) is a senior research fellow in the Environment and Production Technology Division of International Food Policy Research Institute (IFPRI), Washington, DC. Richard Robertson ([email protected]) is a research fellow in the Environment and Production Technology Division of IFPRI, Washington, DC. Salome Begeladze ([email protected]) is a programme officer for Forest Landscape Restoration in the Global Forest and Climate Change Programme of IUCN, Washington, DC. Chetan Kumar ([email protected]) is a manager, Landscape Restoration Science and Knowledge in the Global Forest and Climate Change Programme of IUCN, Washington, DC. Ho-Young Kwon ([email protected]) is a research fellow in the Environment and Production Technology Division of IFPRI, Washington, DC. -

(CONUS) Land Cover Change with Web-Enabled Landsat Data (WELD) M C
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Staff -- ubP lished Research US Geological Survey 2014 Monitoring conterminous United States (CONUS) land cover change with Web-Enabled Landsat Data (WELD) M C. Hansen University of Maryland, College Park, [email protected] A. Egorov South Dakota State University, [email protected] P V. Potapov University of Maryland, College Park S V. Stehman State University of New York A Tyukavina University of Maryland, College Park See next page for additional authors Follow this and additional works at: http://digitalcommons.unl.edu/usgsstaffpub Part of the Earth Sciences Commons, Environmental Sciences Commons, and the Oceanography and Atmospheric Sciences and Meteorology Commons Hansen, M C.; Egorov, A.; Potapov, P V.; Stehman, S V.; Tyukavina, A; Turubanova, S A.; Roy, D. P.; Goetz, S J.; Loveland, T R.; Ju, J; Kommareddy, A.; Kovalskyy, V.; Forsyth, C; and Bents, T, "Monitoring conterminous United States (CONUS) land cover change with Web-Enabled Landsat Data (WELD)" (2014). USGS Staff -- Published Research. 854. http://digitalcommons.unl.edu/usgsstaffpub/854 This Article is brought to you for free and open access by the US Geological Survey at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USGS Staff -- ubP lished Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors M C. Hansen, A. Egorov, P V. Potapov, S V. Stehman, A Tyukavina, S A. Turubanova, D. P. Roy, S J. Goetz, T R. Loveland, J Ju, A. Kommareddy, V. Kovalskyy, C Forsyth, and T Bents This article is available at DigitalCommons@University of Nebraska - Lincoln: http://digitalcommons.unl.edu/usgsstaffpub/854 Remote Sensing of Environment 140 (2014) 466–484 Contents lists available at ScienceDirect Remote Sensing of Environment journal homepage: www.elsevier.com/locate/rse Monitoring conterminous United States (CONUS) land cover change with Web-Enabled Landsat Data (WELD) M.C. -

The Exceptional Value of Intact Forest Ecosystems 6 James E.M
1 Re-submission to Nature Ecology and Evolution as a Perspective 2 3 RE: NATECOLEVOL-17072364A-Z_R3 4 5 The exceptional value of intact forest ecosystems 6 James E.M. Watson1, 2*, Tom Evans2*, Oscar Venter3, Brooke Williams1,2, Ayesha 7 Tulloch1, 2, Claire Stewart1, Ian Thompson4, Justina C. Ray5, Kris Murray6, Alvaro 8 Salazar2, Clive McAlpine2, Peter Potapov7, Joe Walston2, John Robinson2, Michael 9 Painter2, David Wilkie2, Christopher Filardi8, William F. Laurance9, Richard A. 10 Houghton10, Sean Maxwell1, Hedley Grantham1,2, Cristián Samper2, Stephanie 11 Wang2, Lars Laestadius11, Rebecca K. Runting1, Gustavo A. Silva-Chávez12, Jamison 12 Ervin13, David Lindenmayer14 13 1. School of Earth and Environmental Science, The University of Queensland, St. Lucia, Brisbane, 14 Queensland, 4072 Australia 15 16 2. Wildlife Conservation Society, Global Conservation Program, 2300 Southern Boulevard, Bronx, NY 17 10460-1068, USA 18 19 3. Natural Resource and Environmental Studies Institute, University of Northern British Columbia, 20 Prince George, Canada, V2N 2M7 21 22 4. Canadian Forest Service, 1219 Queen St., Sault Ste. Marie, Ontario, Canada 23 24 5. Wildlife Conservation Society Canada, 344 Bloor St. West #204, Toronto, Ontario M5S 3AS7, 25 Canada 26 27 6. Imperial College London, The Grantham Institute - Climate Change and the Environment and 28 Department of Infectious Disease Epidemiology, London, UK 29 30 7. University of Maryland, College Park, MD 20740, USA 31 32 8. Division of Ornithology, American Museum of Natural History, Central Park West at 79th Street, 33 New York USA 10024 34 35 9. Centre for Tropical Environmental and Sustainability Science (TESS) and College of Marine and 36 Environmental Sciences, James Cook University, Cairns, QLD 4878, Australia 37 38 10.