3-6 July 2017 PRAC Meeting Minutes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Commissioning Policy: Susoctocog Alfa for Treating Bleeding Episodes in People with Acquired Haemophilia a (All Ages)
Clinical Commissioning Policy: Susoctocog alfa for treating bleeding episodes in people with acquired haemophilia A (all ages) NHS England Reference: 170061P 1 NHS England INFORMATION READER BOX Directorate Medical Operations and Information Specialised Commissioning Nursing Trans. & Corp. Ops. Commissioning Strategy Finance Publications Gateway Reference: 07603 Document Purpose Policy Clinical commissioning policy: Susoctocog alfa for treating bleeding Document Name episodes in people with acquired haemophilia A (all ages) Author Specialised Commissioning Team Publication Date 29 June 2018 Target Audience CCG Clinical Leaders, Care Trust CEs, Foundation Trust CEs , Medical Directors, Directors of PH, Directors of Nursing, NHS England Regional Directors, NHS England Directors of Commissioning Operations, Directors of Finance, NHS Trust CEs Additional Circulation #VALUE! List Description Routinely Commissioned - NHS England will routinely commission this specialised treatment in accordance with the criteria described in this policy. Cross Reference 0 Superseded Docs 0 (if applicable) Action Required 0 Timing / Deadlines By 00 January 1900 (if applicable) Contact Details for [email protected] further information 0 0 0 0 0 0 Document Status This is a controlled document. Whilst this document may be printed, the electronic version posted on the intranet is the controlled copy. Any printed copies of this document are not controlled. As a controlled document, this document should not be saved onto local or network drives but should always be accessed from the intranet. 2 Standard Operating Procedure: Clinical Commissioning Policy: Susoctocog alfa for treating bleeding episodes in people with acquired haemophilia A (all ages) First published: June 2018 Prepared by the National Institute for Health and Care Excellence (NICE) Commissioning Support Programme Published by NHS England, in electronic format only. -

Could Mycolactone Inspire New Potent Analgesics? Perspectives and Pitfalls
toxins Review Could Mycolactone Inspire New Potent Analgesics? Perspectives and Pitfalls 1 2 3, 4, , Marie-Line Reynaert , Denis Dupoiron , Edouard Yeramian y, Laurent Marsollier * y and 1, , Priscille Brodin * y 1 France Univ. Lille, CNRS, Inserm, CHU Lille, Institut Pasteur de Lille, U1019-UMR8204-CIIL-Center for Infection and Immunity of Lille, F-59000 Lille, France 2 Institut de Cancérologie de l’Ouest Paul Papin, 15 rue André Boquel-49055 Angers, France 3 Unité de Microbiologie Structurale, Institut Pasteur, CNRS, Univ. Paris, F-75015 Paris, France 4 Equipe ATIP AVENIR, CRCINA, INSERM, Univ. Nantes, Univ. Angers, 4 rue Larrey, F-49933 Angers, France * Correspondence: [email protected] (L.M.); [email protected] (P.B.) These three authors contribute equally to this work. y Received: 29 June 2019; Accepted: 3 September 2019; Published: 4 September 2019 Abstract: Pain currently represents the most common symptom for which medical attention is sought by patients. The available treatments have limited effectiveness and significant side-effects. In addition, most often, the duration of analgesia is short. Today, the handling of pain remains a major challenge. One promising alternative for the discovery of novel potent analgesics is to take inspiration from Mother Nature; in this context, the detailed investigation of the intriguing analgesia implemented in Buruli ulcer, an infectious disease caused by the bacterium Mycobacterium ulcerans and characterized by painless ulcerative lesions, seems particularly promising. More precisely, in this disease, the painless skin ulcers are caused by mycolactone, a polyketide lactone exotoxin. In fact, mycolactone exerts a wide range of effects on the host, besides being responsible for analgesia, as it has been shown notably to modulate the immune response or to provoke apoptosis. -

Bonviva, INN- Ibandronic Acid
European Medicines Agency London, 1 September 2005 Doc.Ref.: EMEA/278873/2005 Bonviva International Nonproprietary Name: Ibandronic acid Following the procedure EMEA/H/C/501/X/01 7 Westferry Circus, Canary Wharf, London E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 74 18 86 68 E-mail: [email protected] http://www.emea.eu.int EMEA 2005 Reproduction and/or distribution of this document is authorised for non commercial purposes only provided the EMEA is acknowledged 1 SCIENTIFIC DISCUSSION 1.1 Introduction and rationale The MAH submitted an extension application under Annex II, point 2 iii to Commission Regulation (EC) No 1085/2003 to request the approval of a 150 mg tablet as a monthly dosing regimen of ibandronate only for the indication of “treatment of osteoporosis in postmenopausal women, in order to reduce the risk of vertebral fractures”. This claim is based on the results of a phase III study MOBILE (BM16549) comparing 100 and 150 mg once monthly to 2.5 mg once daily. Due to the inconveniences associated with intake of oral bisphosphonates (i.e. fasting conditions, frequent upper gastrointestinal intolerance) that may result in poor compliance, it was considered desirable to develop a more convenient drug formulation. Hence a 150 mg once monthly oral regimen is expected to offer greater convenience to postmenopausal women when compared to the currently approved 2.5mg once daily tablet. The development programme No new pre-clinical pharmacodynamic and pharmacokinetic studies have been performed in addition to those included in the previous submission for ibandronate 2.5 mg daily oral tablets. -

CCA Senior Care Options Formulary
Commonwealth Care Alliance Senior Care Option HMO SNP 2021 List of Covered Drugs Formulary 30 Winter Street • Boston, MA 02108 PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN This formulary was updated on 08/01/2021. For more recent information or other questions, please contact Senior Care Options Program (HMO SNP) Member Services, at 1-866-610-2273 or, for TTY users, 711, 8 a.m. – 8 p.m., 7 days a week, or visit www.commonwealthcaresco.org. HPMS Approved Formulary File Submission ID 00021589, Version Number 13 Senior Care Options Program (HMO SNP) 2021 Formulary (List of Covered Drugs) PLEASE READ: THIS DO CUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN HPMS Approved Formulary File Submission ID 00021589, Version Number 13 Note to existing members: This formulary has changed since last year. Please review this document to make sure that it still contains the drugs you take. When this drug list (formulary) refers to “we,” “us”, or “our,” it means Commonwealth Care Alliance. When it refers to “plan” or “our plan,” it means 2021 Senior Care Options Program. This document includes list of the drugs (formulary) for our plan which is current as of 08/01/2021. This formulary document applies to all SCO members. For an updated formulary, please contact us. Our contact information, along with the date we last updated the formulary, appears on the front and back cover pages. You must generally use network pharmacies to use your prescription drug benefit. Benefits, formulary, pharmacy n etwork, and/or copayments/coinsurance may change on January 1, 2022, and from time to time during the year. -

Pharmacology – Inhalant Anesthetics
Pharmacology- Inhalant Anesthetics Lyon Lee DVM PhD DACVA Introduction • Maintenance of general anesthesia is primarily carried out using inhalation anesthetics, although intravenous anesthetics may be used for short procedures. • Inhalation anesthetics provide quicker changes of anesthetic depth than injectable anesthetics, and reversal of central nervous depression is more readily achieved, explaining for its popularity in prolonged anesthesia (less risk of overdosing, less accumulation and quicker recovery) (see table 1) Table 1. Comparison of inhalant and injectable anesthetics Inhalant Technique Injectable Technique Expensive Equipment Cheap (needles, syringes) Patent Airway and high O2 Not necessarily Better control of anesthetic depth Once given, suffer the consequences Ease of elimination (ventilation) Only through metabolism & Excretion Pollution No • Commonly administered inhalant anesthetics include volatile liquids such as isoflurane, halothane, sevoflurane and desflurane, and inorganic gas, nitrous oxide (N2O). Except N2O, these volatile anesthetics are chemically ‘halogenated hydrocarbons’ and all are closely related. • Physical characteristics of volatile anesthetics govern their clinical effects and practicality associated with their use. Table 2. Physical characteristics of some volatile anesthetic agents. (MAC is for man) Name partition coefficient. boiling point MAC % blood /gas oil/gas (deg=C) Nitrous oxide 0.47 1.4 -89 105 Cyclopropane 0.55 11.5 -34 9.2 Halothane 2.4 220 50.2 0.75 Methoxyflurane 11.0 950 104.7 0.2 Enflurane 1.9 98 56.5 1.68 Isoflurane 1.4 97 48.5 1.15 Sevoflurane 0.6 53 58.5 2.5 Desflurane 0.42 18.7 25 5.72 Diethyl ether 12 65 34.6 1.92 Chloroform 8 400 61.2 0.77 Trichloroethylene 9 714 86.7 0.23 • The volatile anesthetics are administered as vapors after their evaporization in devices known as vaporizers. -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid. -

Human and Recombinat Coagulation Factor VIII
08 July 2016 EMA/PRAC/471535/2016 PRAC List of questions To be addressed by the marketing authorisation holder(s) for human and recombinant coagulation factor VIII containing medicinal products Referral under Article 31 of Directive 2001/83/EC resulting from pharmacovigilance data Procedure number: EMEA/H/A-31/1448 Advate EMEA/H/C/0520/A31/0078 Elocta EMEA/H/C/3964/A31/0006 Helixate Nexgen EMEA/H/C/0276/A31/0178 Iblias EMEA/H/C/4147/A31/0002 Kogenate EMEA/H/C/0275/A31/0185 Kovaltry EMEA/H/C/3825/A31/0004 Novoeight EMEA/H/C/2719/A31/0014 Nuwiq EMEA/H/C/2813/A31/0015 Obizur EMEA/H/C/2792/A31/0003 Refacto AF EMEA/H/C/0232/A31/0134 Voncento EMEA/H/C/2493/A31/0022 Active substances: human coagulation factor VIII; efmoroctocog alfa; moroctocog alfa; octocog alfa; simoctocog alfa; susoctocog alfa; turoctocog alfa 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2016. Reproduction is authorised provided the source is acknowledged. 1. Background Today’s standard treatment of congenital haemophilia (and acquired haemophilia A) is based on prophylactic or on-demand replacement therapy with coagulation factor VIII (FVIII), either with plasma derived or with recombinant FVIII products. Principally both substance classes may be used for prophylactic treatment as well as for therapeutic treatment in case of spontaneous bleedings. Inhibitor development in haemophilia A patients receiving FVIII products mostly occurs in previously untreated or minimally treated patients (PUPs), who are still within the first 50 days of exposure to the treatment. -

Annual Report 2016
Annexes to the annual report of the European Medicines Agency 2016 Annex 1 – Members of the Management Board ............................................................... 2 Annex 2 - Members of the Committee for Medicinal Products for Human Use ...................... 4 Annex 3 – Members of the Pharmacovigilance Risk Assessment Committee ........................ 6 Annex 4 – Members of the Committee for Medicinal Products for Veterinary Use ................. 8 Annex 5 – Members of the Committee on Orphan Medicinal Products .............................. 10 Annex 6 – Members of the Committee on Herbal Medicinal Products ................................ 12 Annex 7 – Committee for Advanced Therapies .............................................................. 14 Annex 8 – Members of the Paediatric Committee .......................................................... 16 Annex 9 – Working parties and working groups ............................................................ 18 Annex 10 – CHMP opinions: initial evaluations and extensions of therapeutic indication ..... 24 Annex 10a – Guidelines and concept papers adopted by CHMP in 2016 ............................ 25 Annex 11 – CVMP opinions in 2016 on medicinal products for veterinary use .................... 33 Annex 11a – 2016 CVMP opinions on extensions of indication for medicinal products for veterinary use .......................................................................................................... 39 Annex 11b – Guidelines and concept papers adopted by CVMP in 2016 ........................... -

Effects of Alfaxalone Or Propofol on Giant-Breed Dog Neonates Viability
animals Article Effects of Alfaxalone or Propofol on Giant-Breed Dog Neonates Viability During Elective Caesarean Sections Monica Melandri 1, Salvatore Alonge 1,* , Tanja Peric 2, Barbara Bolis 3 and Maria C Veronesi 3 1 Società Veterinaria “Il Melograno” Srl, 21018 Sesto Calende, Varese, Italy; [email protected] 2 Department of Agricultural, Food, Environmental and Animal Sciences, University of Udine, 33100 Udine, Italy; [email protected] 3 Department of Veterinary Medicine, Università degli Studi di Milano, 20100 Milan, Italy; [email protected] (B.B.); [email protected] (M.C.V.) * Correspondence: [email protected]; Tel.: +39-392-805-8524 Received: 20 September 2019; Accepted: 7 November 2019; Published: 13 November 2019 Simple Summary: Nowadays, thanks to the increased awareness of owners and breeders and to the most recent techniques available to veterinarians, the management of parturition, especially of C-sections, has become a topic of greater importance. Anesthesia is crucial and must be targeted to both the mother and neonates. The present study aimed to evaluate the effect of the induction agent alfaxalone on the vitality of puppies born from elective C-section, in comparison to propofol. After inducing general anesthesia for elective C-section, puppies from the mothers induced with alfaxalone had higher 5-min Apgar scores than those induced with propofol. The concentration of cortisol in fetal fluids collected at birth is neither influenced by the anesthetic protocol used, nor does it differ between amniotic and allantoic fluids. Nevertheless, the cortisol concentration in fetal fluids affects the relationship between anesthesia and the Apgar score: the present study highlights a significant relationship between the anesthetic protocol used and Apgar score in puppies, and fetal fluids cortisol concentration acts as a covariate of this relationship. -

Therapeutics Advisory Group
Therapeutics Advisory Group CCG and NHS Trusts in Norfolk and Waveney Index of TAG recommendations Generic name Indication BNFclass Trafficlight IQoro euromuscular training Hiatus hernia - improving symptoms No BNF entry - device Double Red Not recommended for device routine use / Not commissioned (L-) Carnitine Carnitine Deficiency 9.8.1 Drugs used in Red Hospital / Specialist metabolic disorders only (Para-)aminosalicylic acid Tuberculosis 5.1.9 Antituberculosis Double Red Not recommended for drugs - routine use / Not Antimycobacterials commissioned 5-fluorouracil + salicyclic acid Hyperkeratotic actinic keratosis 13.8.1 Photodamage Double-GreenSuitable for GPs to topical solution initiate and prescribe 5-fluorouracil 5% w/w cream Non-hypertrophic actinic keratosis 13.8.1 Photodamage Double-GreenSuitable for GPs to initiate and prescribe Abacavir HIV infection in combination with other 5.3.1 HIV Infection Red Hospital / Specialist antiretroviral drugs only Abacavir + dolutegravir + HIV infection in combination with other 5.3.1 HIV Infection Red Hospital / Specialist lamivudine antiretroviral drugs only Abacavir and lamivudine HIV infection in combination with other 5.3.1 HIV Infection Red Hospital / Specialist antiretroviral drugs only Abaloparatide Male and juvenile osteoporosis 6.6.1 Calcitonin and Double Red Not recommended for Calcitonin and routine use / Not parathyroid hormone commissioned Abatacept Rheumatoid arthritis - 1st line biologic 10.1.3 Drugs that suppress Red Hospital / Specialist after failure of non-biologic DMARDs -

Bisphosphonate Use in Patients with Breast Cancer
Bisphosphonate use in patients with breast cancer Information for patients, relatives and carers For more information, please contact: Medicines Information Email: [email protected] York Hospital Medicines Information Tel: 01904 725960 Page 2 Contents Page Why have I been given this leaflet? ............................... 4 How is the treatment given? .......................................... 4 How long will I need to take the treatment for? .............. 6 Can I take other medicines at the same time? ............... 6 Who will prescribe the medication? ............................... 7 What if I am already on bisphosphonate treatment? ...... 7 Bisphosphonates are not licensed for reducing the risk of breast cancer recurrence. What does this mean? ...... 8 Are there any side effects? ............................................ 9 What is osteonecrosis? ............................................... 10 Are there any signs and symptoms I should look out for during treatment? ........................................................ 11 How can I decrease the risk of developing osteonecrosis of the jaw? ................................................................... 12 What do I need to do before starting treatment? .......... 13 Will I require any monitoring, or blood tests? ............... 14 Should I stop taking the bisphosphonate? ................... 14 Tell us what you think of this leaflet ............................. 15 Teaching, training and research ................................... 15 Patient Advice and Liaison Service (PALS) .................. 15 Page 3 Why have I been given this leaflet? You have been given this leaflet because you are going to be treated with a bisphosphonate medicine (zoledronic acid and/or ibandronic acid). In post-menopausal women, with early breast cancer, these drugs have been shown to reduce the risk of the disease recurring and increase length of life. How is the treatment given? The treatment will usually be provided as an oral tablet of ibandronic acid (one 50mg tablet taken once daily). -
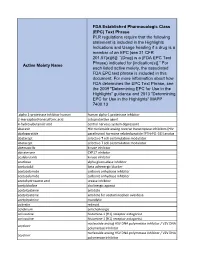
Active Moiety Name FDA Established Pharmacologic Class (EPC) Text
FDA Established Pharmacologic Class (EPC) Text Phrase PLR regulations require that the following statement is included in the Highlights Indications and Usage heading if a drug is a member of an EPC [see 21 CFR 201.57(a)(6)]: “(Drug) is a (FDA EPC Text Phrase) indicated for [indication(s)].” For Active Moiety Name each listed active moiety, the associated FDA EPC text phrase is included in this document. For more information about how FDA determines the EPC Text Phrase, see the 2009 "Determining EPC for Use in the Highlights" guidance and 2013 "Determining EPC for Use in the Highlights" MAPP 7400.13. .alpha.