Supporting Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

From Inverse Agonism to 'Paradoxical Pharmacology' Richard A
International Congress Series 1249 (2003) 27-37 From inverse agonism to 'Paradoxical Pharmacology' Richard A. Bond*, Kenda L.J. Evans, Zsirzsanna Callaerts-Vegh Department of Pharmacological and Pharmaceutical Sciences, University of Houston, 521 Science and Research Bldg 2, 4800 Caltioun, Houston, TX 77204-5037, USA Received 16 April 2003; accepted 16 April 2003 Abstract The constitutive or spontaneous activity of G protein-coupled receptors (GPCRs) and compounds acting as inverse agonists is a recent but well-established phenomenon. Dozens of receptor subtypes for numerous neurotransmitters and hormones have been shown to posses this property. However, do to the apparently low percentage of receptors in the spontaneously active state, the physiologic relevance of these findings remains questionable. The possibility that the reciprocal nature of the effects of agonists and inverse agonists may extend to cellular signaling is discussed, and that this may account for the beneficial effects of certain p-adrenoceptor inverse agonists in the treatment of heart failure. © 2003 Elsevier Science B.V. All rights reserved. Keywords. Inverse agonism; GPCR; Paradoxical pharmacology 1. Brief history of inverse agonism at G protein-coupled receptors For approximately three-quarters of a century, ligands that interacted with G protein- coupled receptors (GPCRs) were classified either as agonists or antagonists. Receptors were thought to exist in a single quiescent state that could only induce cellular signaling upon agonist binding to the receptor to produce an activated state of the receptor. In this model, antagonists had no cellular signaling ability on their own, but did bind to the receptor and prevented agonists from being able to bind and activate the receptor. -
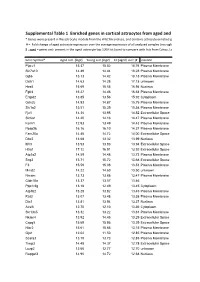
Supplemental Table 1 Enriched Genes in Cortical Astrocytes from Aged
Supplemental Table 1 Enriched genes in cortical astrocytes from aged and young-adult mice * Genes were present in the astrocyte module from the WGCNA analysis, and contains astrocyte enriched genes compared to microglia and oligodendrocytes # = Fold change of aged astrocyte expression over the average expression of all analyzed samples (microglia, astrocytes: young, old, with and without myelin contamination) $ ; aged = genes only present in the aged astrocyte top 1000 list (used to compare with lists from Cahoy, Lovatt, Doyle; see Fig. 4B), all = genes present in all astrocyte top 1000 lists Gene Symbol* Aged astr. (log2) Young astr.(log2) FC (aged/ aver.)# Location Ptprz1 15.37 15.02 18.76 Plasma Membrane Slc7a10 14.49 14.44 18.28 Plasma Membrane Gjb6 15.13 14.42 18.18 Plasma Membrane Dclk1 14.63 14.28 17.18 unknown Hes5 15.69 15.55 16.94 Nucleus Fgfr3 15.27 14.46 16.54 Plasma Membrane Entpd2 13.85 13.56 15.92 Cytoplasm Grin2c 14.93 14.87 15.75 Plasma Membrane Slc1a2 15.51 15.39 15.58 Plasma Membrane Fjx1 14.36 13.98 14.52 Extracellular Space Slc6a1 14.20 14.16 14.47 Plasma Membrane Kcnk1 12.93 13.49 14.43 Plasma Membrane Ppap2b 16.16 16.10 14.37 Plasma Membrane Fam20a 14.48 14.72 14.00 Extracellular Space Dbx2 13.68 13.32 13.99 Nucleus Itih3 13.93 13.93 13.94 Extracellular Space Htra1 17.12 16.91 13.92 Extracellular Space Atp1a2 14.59 14.48 13.73 Plasma Membrane Scg3 15.71 15.72 13.68 Extracellular Space F3 15.59 15.08 13.51 Plasma Membrane Mmd2 14.22 14.60 13.50 unknown Nrcam 13.73 13.88 13.47 Plasma Membrane Cldn10a 13.37 13.57 13.46 -

Podocyte Specific Knockdown of Klf15 in Podocin-Cre Klf15flox/Flox Mice Was Confirmed
SUPPLEMENTARY FIGURE LEGENDS Supplementary Figure 1: Podocyte specific knockdown of Klf15 in Podocin-Cre Klf15flox/flox mice was confirmed. (A) Primary glomerular epithelial cells (PGECs) were isolated from 12-week old Podocin-Cre Klf15flox/flox and Podocin-Cre Klf15+/+ mice and cultured at 37°C for 1 week. Real-time PCR was performed for Nephrin, Podocin, Synaptopodin, and Wt1 mRNA expression (n=6, ***p<0.001, Mann-Whitney test). (B) Real- time PCR was performed for Klf15 mRNA expression (n=6, *p<0.05, Mann-Whitney test). (C) Protein was also extracted and western blot analysis for Klf15 was performed. The representative blot of three independent experiments is shown in the top panel. The bottom panel shows the quantification of Klf15 by densitometry (n=3, *p<0.05, Mann-Whitney test). (D) Immunofluorescence staining for Klf15 and Wt1 was performed in 12-week old Podocin-Cre Klf15flox/flox and Podocin-Cre Klf15+/+ mice. Representative images from four mice in each group are shown in the left panel (X 20). Arrows show colocalization of Klf15 and Wt1. Arrowheads show a lack of colocalization. Asterisk demonstrates nonspecific Wt1 staining. “R” represents autofluorescence from RBCs. In the right panel, a total of 30 glomeruli were selected in each mouse and quantification of Klf15 staining in the podocytes was determined by the ratio of Klf15+ and Wt1+ cells to Wt1+ cells (n=6 mice, **p<0.01, unpaired t test). Supplementary Figure 2: LPS treated Podocin-Cre Klf15flox/flox mice exhibit a lack of recovery in proteinaceous casts and tubular dilatation after DEX administration. -

Defining Functional Interactions During Biogenesis of Epithelial Junctions
ARTICLE Received 11 Dec 2015 | Accepted 13 Oct 2016 | Published 6 Dec 2016 | Updated 5 Jan 2017 DOI: 10.1038/ncomms13542 OPEN Defining functional interactions during biogenesis of epithelial junctions J.C. Erasmus1,*, S. Bruche1,*,w, L. Pizarro1,2,*, N. Maimari1,3,*, T. Poggioli1,w, C. Tomlinson4,J.Lees5, I. Zalivina1,w, A. Wheeler1,w, A. Alberts6, A. Russo2 & V.M.M. Braga1 In spite of extensive recent progress, a comprehensive understanding of how actin cytoskeleton remodelling supports stable junctions remains to be established. Here we design a platform that integrates actin functions with optimized phenotypic clustering and identify new cytoskeletal proteins, their functional hierarchy and pathways that modulate E-cadherin adhesion. Depletion of EEF1A, an actin bundling protein, increases E-cadherin levels at junctions without a corresponding reinforcement of cell–cell contacts. This unexpected result reflects a more dynamic and mobile junctional actin in EEF1A-depleted cells. A partner for EEF1A in cadherin contact maintenance is the formin DIAPH2, which interacts with EEF1A. In contrast, depletion of either the endocytic regulator TRIP10 or the Rho GTPase activator VAV2 reduces E-cadherin levels at junctions. TRIP10 binds to and requires VAV2 function for its junctional localization. Overall, we present new conceptual insights on junction stabilization, which integrate known and novel pathways with impact for epithelial morphogenesis, homeostasis and diseases. 1 National Heart and Lung Institute, Faculty of Medicine, Imperial College London, London SW7 2AZ, UK. 2 Computing Department, Imperial College London, London SW7 2AZ, UK. 3 Bioengineering Department, Faculty of Engineering, Imperial College London, London SW7 2AZ, UK. 4 Department of Surgery & Cancer, Faculty of Medicine, Imperial College London, London SW7 2AZ, UK. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Mice Carrying a Human GLUD2 Gene Recapitulate Aspects of Human Transcriptome and Metabolome Development
Mice carrying a human GLUD2 gene recapitulate aspects of human transcriptome and metabolome development Qian Lia,b,1, Song Guoa,1, Xi Jianga, Jaroslaw Brykc,2, Ronald Naumannd, Wolfgang Enardc,3, Masaru Tomitae, Masahiro Sugimotoe, Philipp Khaitovicha,c,f,4, and Svante Pääboc,4 aChinese Academy of Sciences Key Laboratory of Computational Biology, Chinese Academy of Sciences-Max Planck Partner Institute for Computational Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, 200031 Shanghai, China; bUniversity of Chinese Academy of Sciences, 100049 Beijing, China; cMax Planck Institute for Evolutionary Anthropology, 04103 Leipzig, Germany; dMax Planck Institute of Molecular Cell Biology and Genetics, D-01307 Dresden, Germany; eInstitute for Advanced Biosciences, Keio University, 997-0035 Tsuruoka, Yamagata, Japan; and fSkolkovo Institute for Science and Technology, 143025 Skolkovo, Russia Edited by Joshua M. Akey, University of Washington, Seattle, WA, and accepted by the Editorial Board April 1, 2016 (received for review September 28, 2015) Whereas all mammals have one glutamate dehydrogenase gene metabolic flux from glucose and glutamine to lipids by way of the (GLUD1), humans and apes carry an additional gene (GLUD2), TCA cycle (12). which encodes an enzyme with distinct biochemical properties. To investigate the physiological role the GLUD2 gene may We inserted a bacterial artificial chromosome containing the human play in human and ape brains, we generated mice transgenic for GLUD2. GLUD2 gene into mice and analyzed the resulting changes in the a genomic region containing human We compared effects transcriptome and metabolome during postnatal brain development. on gene expression and metabolism during postnatal development Effects were most pronounced early postnatally, and predominantly of the frontal cortex of the brain in these mice and their wild-type genes involved in neuronal development were affected. -

Large Sex Differences in Chicken Behavior and Brain Gene Expression Coincide with Few Differences in Promoter DNA-Methylation
Large Sex Differences in Chicken Behavior and Brain Gene Expression Coincide with Few Differences in Promoter DNA-Methylation Daniel Na¨tt1,2*, Beatrix Agnvall1, Per Jensen1 1 IFM Biology, AVIAN Behaviour and Genomics group, Linko¨ping University, Linko¨ping, Sweden, 2 Department of Clinical and Experimental Medicine, Laboratory of Integrative and Behavioral Neuroscience, Linko¨ping University, Linko¨ping, Sweden Abstract While behavioral sex differences have repeatedly been reported across taxa, the underlying epigenetic mechanisms in the brain are mostly lacking. Birds have previously shown to have only limited dosage compensation, leading to high sex bias of Z-chromosome gene expression. In chickens, a male hyper-methylated region (MHM) on the Z-chromosome has been associated with a local type of dosage compensation, but a more detailed characterization of the avian methylome is limiting our interpretations. Here we report an analysis of genome wide sex differences in promoter DNA-methylation and gene expression in the brain of three weeks old chickens, and associated sex differences in behavior of Red Junglefowl (ancestor of domestic chickens). Combining DNA-methylation tiling arrays with gene expression microarrays we show that a specific locus of the MHM region, together with the promoter for the zinc finger RNA binding protein (ZFR) gene on chromosome 1, is strongly associated with sex dimorphism in gene expression. Except for this, we found few differences in promoter DNA-methylation, even though hundreds of genes were robustly differentially expressed across distantly related breeds. Several of the differentially expressed genes are known to affect behavior, and as suggested from their functional annotation, we found that female Red Junglefowl are more explorative and fearful in a range of tests performed throughout their lives. -

Guthrie Cdna Resource Center
cDNA Resource Center cDNA Resource Center Catalog cDNA Resource Center Missouri University of Science and Technology 400 W 11th Rolla, MO 65409 TEL: (573) 341-7610 FAX: (573) 341-7609 EMAIL: [email protected] www.cdna.org September, 2008 1 cDNA Resource Center Visit our web site for product updates 2 cDNA Resource Center The cDNA Resource Center The cDNA Resource Center is a service provided by the faculty of the Department of Biological Sciences of Missouri University of Science and Technology. The purpose of the cDNA Resource Center is to further scientific investigation by providing cDNA clones of human proteins involved in signal transduction processes. This is achieved by providing high quality clones for important signaling proteins in a timely manner. By high quality, we mean that the clones are • Sequence verified • Propagated in a versatile vector useful in bacterial and mammalian systems • Free of extraneous 3' and 5' untranslated regions • Expression verified (in most cases) by coupled in vitro transcription/translation assays • Available in wild-type, epitope-tagged and common mutant forms (e.g., constitutively- active or dominant negative) By timely, we mean that the clones are • Usually shipped within a day from when you place your order. Clones can be ordered from our web pages, by FAX or by phone. Within the United States, clones are shipped by overnight courier (FedEx); international orders are shipped International Priority (FedEx). The clones are supplied for research purposes only. Details on use of the material are included on the Material Transfer Agreement (page 3). Clones are distributed by agreement in Invitrogen's pcDNA3.1+ vector. -

Supp Material.Pdf
Simon et al. Supplementary information: Table of contents p.1 Supplementary material and methods p.2-4 • PoIy(I)-poly(C) Treatment • Flow Cytometry and Immunohistochemistry • Western Blotting • Quantitative RT-PCR • Fluorescence In Situ Hybridization • RNA-Seq • Exome capture • Sequencing Supplementary Figures and Tables Suppl. items Description pages Figure 1 Inactivation of Ezh2 affects normal thymocyte development 5 Figure 2 Ezh2 mouse leukemias express cell surface T cell receptor 6 Figure 3 Expression of EZH2 and Hox genes in T-ALL 7 Figure 4 Additional mutation et deletion of chromatin modifiers in T-ALL 8 Figure 5 PRC2 expression and activity in human lymphoproliferative disease 9 Figure 6 PRC2 regulatory network (String analysis) 10 Table 1 Primers and probes for detection of PRC2 genes 11 Table 2 Patient and T-ALL characteristics 12 Table 3 Statistics of RNA and DNA sequencing 13 Table 4 Mutations found in human T-ALLs (see Fig. 3D and Suppl. Fig. 4) 14 Table 5 SNP populations in analyzed human T-ALL samples 15 Table 6 List of altered genes in T-ALL for DAVID analysis 20 Table 7 List of David functional clusters 31 Table 8 List of acquired SNP tested in normal non leukemic DNA 32 1 Simon et al. Supplementary Material and Methods PoIy(I)-poly(C) Treatment. pIpC (GE Healthcare Lifesciences) was dissolved in endotoxin-free D-PBS (Gibco) at a concentration of 2 mg/ml. Mice received four consecutive injections of 150 μg pIpC every other day. The day of the last pIpC injection was designated as day 0 of experiment. -

Identification of Transcriptomic Differences Between Lower
International Journal of Molecular Sciences Article Identification of Transcriptomic Differences between Lower Extremities Arterial Disease, Abdominal Aortic Aneurysm and Chronic Venous Disease in Peripheral Blood Mononuclear Cells Specimens Daniel P. Zalewski 1,*,† , Karol P. Ruszel 2,†, Andrzej St˛epniewski 3, Dariusz Gałkowski 4, Jacek Bogucki 5 , Przemysław Kołodziej 6 , Jolanta Szyma ´nska 7 , Bartosz J. Płachno 8 , Tomasz Zubilewicz 9 , Marcin Feldo 9,‡ , Janusz Kocki 2,‡ and Anna Bogucka-Kocka 1,‡ 1 Chair and Department of Biology and Genetics, Medical University of Lublin, 4a Chod´zkiSt., 20-093 Lublin, Poland; [email protected] 2 Chair of Medical Genetics, Department of Clinical Genetics, Medical University of Lublin, 11 Radziwiłłowska St., 20-080 Lublin, Poland; [email protected] (K.P.R.); [email protected] (J.K.) 3 Ecotech Complex Analytical and Programme Centre for Advanced Environmentally Friendly Technologies, University of Marie Curie-Skłodowska, 39 Gł˛ebokaSt., 20-612 Lublin, Poland; [email protected] 4 Department of Pathology and Laboratory Medicine, Rutgers-Robert Wood Johnson Medical School, One Robert Wood Johnson Place, New Brunswick, NJ 08903-0019, USA; [email protected] 5 Chair and Department of Organic Chemistry, Medical University of Lublin, 4a Chod´zkiSt., Citation: Zalewski, D.P.; Ruszel, K.P.; 20-093 Lublin, Poland; [email protected] St˛epniewski,A.; Gałkowski, D.; 6 Laboratory of Diagnostic Parasitology, Chair and Department of Biology and Genetics, Medical University of Bogucki, J.; Kołodziej, P.; Szyma´nska, Lublin, 4a Chod´zkiSt., 20-093 Lublin, Poland; [email protected] J.; Płachno, B.J.; Zubilewicz, T.; Feldo, 7 Department of Integrated Paediatric Dentistry, Chair of Integrated Dentistry, Medical University of Lublin, M.; et al. -

Differential Gene Expression in Oligodendrocyte Progenitor Cells, Oligodendrocytes and Type II Astrocytes
Tohoku J. Exp. Med., 2011,Differential 223, 161-176 Gene Expression in OPCs, Oligodendrocytes and Type II Astrocytes 161 Differential Gene Expression in Oligodendrocyte Progenitor Cells, Oligodendrocytes and Type II Astrocytes Jian-Guo Hu,1,2,* Yan-Xia Wang,3,* Jian-Sheng Zhou,2 Chang-Jie Chen,4 Feng-Chao Wang,1 Xing-Wu Li1 and He-Zuo Lü1,2 1Department of Clinical Laboratory Science, The First Affiliated Hospital of Bengbu Medical College, Bengbu, P.R. China 2Anhui Key Laboratory of Tissue Transplantation, Bengbu Medical College, Bengbu, P.R. China 3Department of Neurobiology, Shanghai Jiaotong University School of Medicine, Shanghai, P.R. China 4Department of Laboratory Medicine, Bengbu Medical College, Bengbu, P.R. China Oligodendrocyte precursor cells (OPCs) are bipotential progenitor cells that can differentiate into myelin-forming oligodendrocytes or functionally undetermined type II astrocytes. Transplantation of OPCs is an attractive therapy for demyelinating diseases. However, due to their bipotential differentiation potential, the majority of OPCs differentiate into astrocytes at transplanted sites. It is therefore important to understand the molecular mechanisms that regulate the transition from OPCs to oligodendrocytes or astrocytes. In this study, we isolated OPCs from the spinal cords of rat embryos (16 days old) and induced them to differentiate into oligodendrocytes or type II astrocytes in the absence or presence of 10% fetal bovine serum, respectively. RNAs were extracted from each cell population and hybridized to GeneChip with 28,700 rat genes. Using the criterion of fold change > 4 in the expression level, we identified 83 genes that were up-regulated and 89 genes that were down-regulated in oligodendrocytes, and 92 genes that were up-regulated and 86 that were down-regulated in type II astrocytes compared with OPCs. -
Title Flap Loop of Glud2 Binds to Cbln1 and Induces Presynaptic
Flap loop of GluD2 binds to Cbln1 and induces presynaptic Title differentiation Author(s) Kuroyanagi, Tomoaki; Hirano, Tomoo Biochemical and biophysical research communications (2010), Citation 398(3): 537-541 Issue Date 2010-07-30 URL http://hdl.handle.net/2433/128851 © 2010 Elsevier Inc.; This is not the published version. Please cite only the published version.; この論文は出版社版であり Right ません。引用の際には出版社版をご確認ご利用ください 。 Type Journal Article Textversion author Kyoto University Flap loop of GluD2 binds to Cbln1 and induces presynaptic differentiation Tomoaki Kuroyanagi and Tomoo Hirano Department of Biophysics, Graduate School of Science, Kyoto University, Kyoto 606-8502, Japan Department of Biophysics, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan Correspondence should be addressed to: T. Hirano Tel, 81-75-753-4237 Fax, 81-75-753-4227 E-mail, [email protected] (Tomoaki Kuroyanagi); [email protected] (Tomoo Hirano) 1 Abstract Glutamate receptor δ2 (GluD2) is selectively expressed on the postsynaptic spines at parallel-fiber (PF)-Purkinje neuron (PN) synapses. GluD2 knockout mice show a reduced number of PF-PN synapses, suggesting that GluD2 is involved in synapse formation. Recent studies revealed that GluD2 induces presynaptic differentiation in a manner dependent on its N-terminal domain (NTD) through binding of Cbln1 secreted from cerebellar granule neurons. However, the underlying mechanism of the specific binding of the NTD to Cbln1 remains elusive. Here, we have identified the flap loop (Arg321-Trp339) in the NTD of GluD2 (GluD2-NTD) as a crucial region for the binding to Cbln1 and the induction of presynaptic differentiation.