The Turquoise-Chalcosiderite Cu(Al,Fe3+)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phase Equilibria and Thermodynamic Properties of Minerals in the Beo
American Mineralogist, Volwne 71, pages 277-300, 1986 Phaseequilibria and thermodynamic properties of mineralsin the BeO-AlrO3-SiO2-H2O(BASH) system,with petrologicapplications Mlnx D. B.qnroN Department of Earth and SpaceSciences, University of California, Los Angeles,Los Angeles,California 90024 Ansrru,cr The phase relations and thermodynamic properties of behoite (Be(OH)r), bertrandite (BeoSirOr(OH)J, beryl (BerAlrSiuO,r),bromellite (BeO), chrysoberyl (BeAl,Oo), euclase (BeAlSiOo(OH)),and phenakite (BerSiOo)have been quantitatively evaluatedfrom a com- bination of new phase-equilibrium, solubility, calorimetric, and volumetric measurements and with data from the literature. The resulting thermodynamic model is consistentwith natural low-variance assemblagesand can be used to interpret many beryllium-mineral occurTences. Reversedhigh-pressure solid-media experimentslocated the positions of four reactions: BerAlrSiuO,,: BeAlrOo * BerSiOo+ 5SiO, (dry) 20BeAlSiOo(OH): 3BerAlrsi6or8+ TBeAlrOo+ 2BerSiOn+ l0HrO 4BeAlSiOo(OH)+ 2SiOr: BerAlrSiuO,,+ BeAlrOo+ 2H2O BerAlrSiuO,,+ 2AlrSiOs : 3BeAlrOa + 8SiO, (water saturated). Aqueous silica concentrationswere determined by reversedexperiments at I kbar for the following sevenreactions: 2BeO + H4SiO4: BerSiOo+ 2H2O 4BeO + 2HoSiOo: BeoSirO'(OH),+ 3HrO BeAlrOo* BerSiOo+ 5H4Sio4: Be3AlrSiuOr8+ loHro 3BeAlrOo+ 8H4SiO4: BerAlrSiuOrs+ 2AlrSiO5+ l6HrO 3BerSiOo+ 2AlrSiO5+ 7H4SiO4: 2BerAlrSiuOr8+ l4H2o aBeAlsioloH) + Bersio4 + 7H4sio4:2BerAlrsiuors + 14Hro 2BeAlrOo+ BerSiOo+ 3H4SiOo: 4BeAlSiOr(OH)+ 4HrO. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Pyrophyllite Solid Solutions in the System A|,O,-S|O,-H,O
American Minerulogist, Volume 59, pages 254-260, 1974 PyrophylliteSolid Solutions in the SystemA|,O,-S|O,-H,O Pnrr,rp E. RoseNnenc D ep art ment ol G eolo gy, 14as hing ton Stat e U nio er sity, P ullman, 14as hington 99 I 6 3 Abstract Pyrophyllite solid solutions have been synthesized in the system Al,O'-SiOr-H,O between 400' and 565'C at 2 kbar from a variety of starting materials using sealed gold tubes and conventional hydrothermal techniques. The duration of experiments was 3-9 weeks. Products were characterized by X-ray measurementsand by infrared spectroscopy. Appreciably larger basal spacings were observed for pyrophyllite synthesized from gels than for natural pyrophyllite; small amounts of quartz were present in these samples. Basal spacings of pyrophyllite synthesized from mixtures of kaolinite and quartz approach but do not equal those of natural pyrophyllite. Rehydroxylation of dehydroxylated pyrophyllite under hydrothermal conditions suggests that enlargement of basal spacings cannot be due to dehydroxylation. Substitution of the type Al"* + H* : Sia* is proposed to explain the observed variations. The coupled substitution of Al3* and (OH)- for Sin*and O- results in expanded basal spacingsdue largely to the formation of OH on the basal surface and in increased thermal stability due to the formation of H-bonds between oxygens in adjacent silica sheets.Analyses of natural pyrophyllite also suggest limited substitution of this type. Failure to recognize these substitutions in synthetic samples accounts, in part, for dis- crepancies in the reported thermal stability of pyrophyllite. Phase characterization, often lack- ing, is essentialin experimentalstudies of mineral stabilities. -

Personal Body Ornamentation on the Southern Iberian Meseta: an Archaeomineralogical Study
Journal of Archaeological Science: Reports 5 (2016) 156–167 Contents lists available at ScienceDirect Journal of Archaeological Science: Reports journal homepage: www.elsevier.com/locate/jasrep Personal body ornamentation on the Southern Iberian Meseta: An archaeomineralogical study Carlos P. Odriozola a,⁎, Luis Benítez de Lugo Enrich b,c, Rodrigo Villalobos García c, José M. Martínez-Blanes d, Miguel A. Avilés e, Norberto Palomares Zumajo f, María Benito Sánchez g, Carlos Barrio Aldea h, Domingo C. Salazar-García i,j a Dpto. de Prehistoria y Arqueología, Universidad de Sevilla, Spain b Dpto. de Prehistoria y Arqueología, Universidad Autónoma de Madrid, Spain c Dpto. de Prehistoria y Arqueología, Centro asociado UNED-Ciudad Real, Universidad Nacional de Educación a Distancia, Spain d Dpto. de Prehistoria, Arqueología, Antropología Social y Ciencias y Técnicas Historiográficas, Universidad de Valladolid, Spain e Instituto de Ciencia de Materiales de Sevilla, Centro mixto Universidad de Sevilla—CSIC, Spain f Anthropos, s.l., Spain g Laboratorio de Antropología Forense, Universidad Complutense de Madrid, Spain h Archaeologist i Department of Archaeology, University of Capetown, South Africa j Departament de Prehistòria i Arqueologia, Universitat de València, Spain article info abstract Article history: Beads and pendants from the Castillejo del Bonete (Terrinches, Ciudad Real) and Cerro Ortega (Villanueva de la Received 22 June 2015 Fuente, Ciudad Real) burials were analysed using XRD, micro-Raman and XRF in order to contribute to the cur- Received in revised form 30 October 2015 rent distribution map of green bead body ornament pieces on the Iberian Peninsula which, so far, remain Accepted 14 November 2015 undetailed for many regions. -

The Pyrophyllite Deposits of North Carolina
North Carolina Department of Conservation and Development Wade H. Phillips, Director BULLETIN NUMBER 37 THE PYROPHYLLITE DEPOSITS OF NORTH CAROLINA With A MORE DETAILED ACCOUNT OF THE GEOLOGY OF THE DEEP RIVER REGION By JASPER L. STUCKEY, Ph. D. RALEIGH 19 28 MEMBERS OF THE BOARD OF CONSERVATION AND DEVELOPMENT Governor A. W. McLean, Chairman, ex offtcio Raleigh S. Wade Marr __._ Raleigh B. B. Gossett Charlotte Jas. G. K. McClure, Jr Asheville Fred I. Sutton ~ _: .. Kinston E. D. Cranford . Asheboro R. Bruce Btheridge Manteo Santford Martin . ; Winston-Salem E. S. Askew Merry Hill J. Q. Gilket Marion F. S. Worthy Washington George L. Hampton Canton Frank H. Stedman Fayetteville Wade H. Phillips, Director, Raleigh LETTER OF TRANSMITTAL Raleigh, N. C, August 1, 1928. To His Excellency, Hon. A. W. McLean, Governor of North Carolina. Sir:—I herewith submit for publication as Bulletin No. 37 of the publications of the North Carolina Department of Con servation and Development a report on The Pyrophyllite De posits of North Carolina, which has been prepared by Dr. Jasper L. Stuckey, former State Geologist. This is the first complete report on the pyrophyllite deposits of this State and will, therefore, be of great interest to those who desire infor mation on such material. Yours respectfully, Wade H. Phillips, Director, North Carolina Department of Conservation and Development. AUTHOR'S FOREWORD This report was begun as a special investigation of the geology and pyrophyllite deposits of the Deep River Region, and accord ingly a detailed geologic map of that region was prepared. As the work progressed, other pyrophyllite deposits were found outside the area mapped. -

Thirty-Seventh List of New Mineral Names. Part 1" A-L
Thirty-seventh list of new mineral names. Part 1" A-L A. M. CLARK Department of Mineralogy, The Natural History Museum, Cromwell Road, London SW7 5BD, UK AND V. D. C. DALTRYt Department of Geology and Mineralogy, University of Natal, Private Bag XO1, Scottsville, Pietermaritzburg 3209, South Africa THE present list is divided into two sections; the pegmatites at Mount Alluaiv, Lovozero section M-Z will follow in the next issue. Those Complex, Kola Peninsula, Russia. names representing valid species, accredited by the Na19(Ca,Mn)6(Ti,Nb)3Si26074C1.H20. Trigonal, IMA Commission on New Minerals and Mineral space group R3m, a 14.046, c 60.60 A, Z = 6. Names, are shown in bold type. Dmeas' 2.76, Dc~ac. 2.78 g/cm3, co 1.618, ~ 1.626. Named for the locality. Abenakiite-(Ce). A.M. McDonald, G.Y. Chat and Altisite. A.P. Khomyakov, G.N. Nechelyustov, G. J.D. Grice. 1994. Can. Min. 32, 843. Poudrette Ferraris and G. Ivalgi, 1994. Zap. Vses. Min. Quarry, Mont Saint-Hilaire, Quebec, Canada. Obschch., 123, 82 [Russian]. Frpm peralkaline Na26REE(SiO3)6(P04)6(C03)6(S02)O. Trigonal, pegmatites at Oleny Stream, SE Khibina alkaline a 16.018, c 19.761 A, Z = 3. Named after the massif, Kola Peninsula, Russia. Monoclinic, a Abenaki Indian tribe. 10.37, b 16.32, c 9.16 ,~, l~ 105.6 ~ Z= 2. Named Abswurmbachite. T. Reinecke, E. Tillmanns and for the chemical elements A1, Ti and Si. H.-J. Bernhardt, 1991. Neues Jahrb. Min. Abh., Ankangite. M. Xiong, Z.-S. -

Winter 1998 Gems & Gemology
WINTER 1998 VOLUME 34 NO. 4 TABLE OF CONTENTS 243 LETTERS FEATURE ARTICLES 246 Characterizing Natural-Color Type IIb Blue Diamonds John M. King, Thomas M. Moses, James E. Shigley, Christopher M. Welbourn, Simon C. Lawson, and Martin Cooper pg. 247 270 Fingerprinting of Two Diamonds Cut from the Same Rough Ichiro Sunagawa, Toshikazu Yasuda, and Hideaki Fukushima NOTES AND NEW TECHNIQUES 281 Barite Inclusions in Fluorite John I. Koivula and Shane Elen pg. 271 REGULAR FEATURES 284 Gem Trade Lab Notes 290 Gem News 303 Book Reviews 306 Gemological Abstracts 314 1998 Index pg. 281 pg. 298 ABOUT THE COVER: Blue diamonds are among the rarest and most highly valued of gemstones. The lead article in this issue examines the history, sources, and gemological characteristics of these diamonds, as well as their distinctive color appearance. Rela- tionships between their color, clarity, and other properties were derived from hundreds of samples—including such famous blue diamonds as the Hope and the Blue Heart (or Unzue Blue)—that were studied at the GIA Gem Trade Laboratory over the past several years. The diamonds shown here range from 0.69 to 2.03 ct. Photo © Harold & Erica Van Pelt––Photographers, Los Angeles, California. Color separations for Gems & Gemology are by Pacific Color, Carlsbad, California. Printing is by Fry Communications, Inc., Mechanicsburg, Pennsylvania. © 1998 Gemological Institute of America All rights reserved. ISSN 0016-626X GIA “Cut” Report Flawed? The long-awaited GIA report on the ray-tracing analysis of round brilliant diamonds appeared in the Fall 1998 Gems & Gemology (“Modeling the Appearance of the Round Brilliant Cut Diamond: An Analysis of Brilliance,” by T. -

The Turquoise-Chalcosiderite-Planerite
СПИСАНИЕ НА БЪЛГАРСКОТО ГЕОЛОГИЧЕСКО ДРУЖЕСТВО, год. 80, кн. 3, 2019, с. 48–50 REVIEW OF THE BULGARIAN GEOLOGICAL SOCIETY, vol. 80, part 3, 2019, p. 48–50 Национална конференция с международно участие „ГЕОНАУКИ 2019“ National Conference with international participation “GEOSCIENCES 2019” The turquoise-chalcosiderite-planerite solid-solution series in samples from Chala deposit, Eastern Rhodopes Тюркоаз-халкосидерит-планеритова серия от твърди разтвори в образци от находище Чала, Източни Родопи Yana Tzvetanova1, Louiza Dimowa1, Elena Tacheva1, Iskra Piroeva2, Ognyan Petrov1, Aleksandar Nikolov1 Яна Цветанова1, Луиза Димова1, Елена Тачева1, Искра Пироева2, Огнян Петров1, Александър Николов1 1 Institute of Mineralogy and Crystallography, Bulgarian Academy of Sciences, Acad. G. Bonchev Str., bl. 107, 1113 Sofia, Bulgaria; E-mail: [email protected] 2 Institute of Physical Chemistry, Bulgarian Academy of Sciences, Acad. G. Bonchev Str., bl. 11, 1113 Sofia, Bulgaria Keywords: turquoise, chalcosiderite, planerite, crystal chemistry, phosphates. Introduction quoise was also reported from the Obichnik depos- it, Zvezdel-Pcheloyad ore field, Eastern Rhodopes The turquoise group, as redefined by Foord and (Kunov, Mandova, 1997). Taggart (1998), consists of 6 members: planerite, The present study aims to show the crystal chem- turquoise, faustite, aheylite, chalcosiderite and an istry of green mineral from the turquoise group from 2+ 3+ unnamed Fe –Fe analogue with the general for- Chala deposit (Spahievo ore field) with particular at- mula A0–1B6(PO4)4–x(PO3OH)x(OH)8 4H2O, where tention to planerite end-member that was approved 2+ x = 0–2. Blue turquoise has Cu at the A position and by the IMA CNMMN as a revalidated mineral in 3+ Al at the B position, whereas green⋅ chalcosiderite 1984. -

Minerals of Turquoise Group from Sândominic, Gurghiu Mts., Romania and from Parádfürdő, Mátra Mts., Hungary
Acta Mineralogica-Petrographica, Abstract Series, Szeged, Vol. 7, 2012 133 MINERALS OF TURQUOISE GROUP FROM SÂNDOMINIC, GURGHIU MTS., ROMANIA AND FROM PARÁDFÜRDŐ, MÁTRA MTS., HUNGARY SZAKÁLL, S.*, KRISTÁLY, F. & ZAJZON, N. Institute of Mineralogy and Geology, University of Miskolc, H-3515 Miskolc-Egyetemváros, Hungary * E-mail: [email protected] 1) The Sândominic occurrence (Dorma Hill) is lo- with the sulphide-rich plutons at depth. This interaction cated in the southern termination of Gurghiu Mts, East- with Cu-Zn-Fe-rich sulphides produced acidic fluids ern Carpathians, Romania, in the vicinity (~5 km from with leached out ions (Cu-Zn-Fe), which were necessary Fagul Cetăţii deposit) of the Bălan copper ore minerali- to form turquoise group minerals. The source of phos- zation. The site is located on the contact of the Rebra phorus could be the hydrothermally altered rock- metamorphic limestones and Tulgheş Lithogroup. Tur- forming apatite. Here the turquoise mineral (Fig. 1) is a 2+ quoise (Fig. 1) was found as incrustations in a highly solid solution of aheylite (Fe ,Zn)Al6(PO4)4(OH)8 • fractured and oxidized, quartz dominated part of a milo- 4H2O, faustite (Zn,Cu)Al6(PO4)4(OH)8 • 4H2O and nitic rock. In the cracks and voids of quartz it is associ- planerite Al6(PO4)2(PO3OH)2(OH)8 • 4H2O. Similar ated with goethite, occasionally with mm-size euhedral situation was already observed elsewhere (FOORD & quartz. Here the meteoric fluids permeated the meta- TAGGERT, 1998). The mineral appears as pale yellow- morphic rocks creating oxidizing environment, where ish brown hemispheres, up to 0.5 mm in diameter. -

Structures of the 2:1 Layers of Pyrophyllite and Talc
Clays and Clay Minerals, Vol. 60, No. 6, 574–587, 2012. STRUCTURES OF THE 2:1 LAYERS OF PYROPHYLLITE AND TALC 1 2 1, 3 V ICTOR A. DRITS ,STEPHEN G UGGENHEIM ,BELLA B. ZVIAGINA *, AND T OSHIHIRO K OGURE 1 Geological Institute of the Russian Academy of Science, Pyzhevsky per. 7, 119017 Moscow, Russia 2 Department of Earth and Environmental Sciences, University of Illinois at Chicago, Chicago, IL 60607, USA 3 Department of Earth and Planetary Science, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-0033, Japan Abstract—To determine the relationships between the symmetry of the overall pyrophyllite and talc structure and the symmetry of individual layers, the geometry and symmetry of each 2:1 layer of pyrophyllite and talc were analyzed. For each, the previously published, refined unit cell may be rotated clockwise by ~60º for comparison to a layer unit cell. In pyrophyllite, the layer unit cell is ideal and shown to be orthogonal with C2/m symmetry. The agreement between the refined atomic coordinates and those calculated for the layer with C2/m symmetry confirms that the symmetry of the pyrophyllite layer is C2/m. The obliquity of the pyrophyllite refined cell results from the layer stacking and the choice of unit cell, but the interlayer stacking sequence does not disturb the layer symmetry. In contrast, talc has an oblique layer cell, without a mirror plane. For the most part, the distortion of the talc 2:1 layer is probably caused by an elongation of unshared OÀO lateral edges around M1 that creates a slight corrugation of the octahedral sheet surface. -

Minerals Found in Michigan Listed by County
Michigan Minerals Listed by Mineral Name Based on MI DEQ GSD Bulletin 6 “Mineralogy of Michigan” Actinolite, Dickinson, Gogebic, Gratiot, and Anthonyite, Houghton County Marquette counties Anthophyllite, Dickinson, and Marquette counties Aegirinaugite, Marquette County Antigorite, Dickinson, and Marquette counties Aegirine, Marquette County Apatite, Baraga, Dickinson, Houghton, Iron, Albite, Dickinson, Gratiot, Houghton, Keweenaw, Kalkaska, Keweenaw, Marquette, and Monroe and Marquette counties counties Algodonite, Baraga, Houghton, Keweenaw, and Aphrosiderite, Gogebic, Iron, and Marquette Ontonagon counties counties Allanite, Gogebic, Iron, and Marquette counties Apophyllite, Houghton, and Keweenaw counties Almandite, Dickinson, Keweenaw, and Marquette Aragonite, Gogebic, Iron, Jackson, Marquette, and counties Monroe counties Alunite, Iron County Arsenopyrite, Marquette, and Menominee counties Analcite, Houghton, Keweenaw, and Ontonagon counties Atacamite, Houghton, Keweenaw, and Ontonagon counties Anatase, Gratiot, Houghton, Keweenaw, Marquette, and Ontonagon counties Augite, Dickinson, Genesee, Gratiot, Houghton, Iron, Keweenaw, Marquette, and Ontonagon counties Andalusite, Iron, and Marquette counties Awarurite, Marquette County Andesine, Keweenaw County Axinite, Gogebic, and Marquette counties Andradite, Dickinson County Azurite, Dickinson, Keweenaw, Marquette, and Anglesite, Marquette County Ontonagon counties Anhydrite, Bay, Berrien, Gratiot, Houghton, Babingtonite, Keweenaw County Isabella, Kalamazoo, Kent, Keweenaw, Macomb, Manistee, -
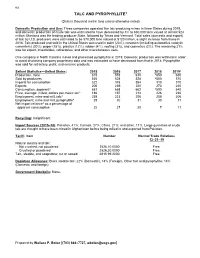
Talc Data Sheet
162 TALC AND PYROPHYLLITE1 (Data in thousand metric tons unless otherwise noted) Domestic Production and Use: Three companies operated five talc-producing mines in three States during 2019, and domestic production of crude talc was estimated to have decreased by 3% to 630,000 tons valued at almost $24 million. Montana was the leading producer State, followed by Texas and Vermont. Total sales (domestic and export) of talc by U.S. producers were estimated to be 570,000 tons valued at $120 million, a slight increase from those in 2018. Talc produced and sold in the United States was used in paint (23%), ceramics (including automotive catalytic converters) (20%), paper (15%), plastics (12%), rubber (4%), roofing (3%), and cosmetics (2%). The remaining 21% was for export, insecticides, refractories, and other miscellaneous uses. One company in North Carolina mined and processed pyrophyllite in 2019. Domestic production was withheld in order to avoid disclosing company proprietary data and was estimated to have decreased from that in 2018. Pyrophyllite was sold for refractory, paint, and ceramic products. Salient Statistics—United States: 2015 2016 2017 2018 2019e Production, mine 615 578 610 e650 630 Sold by producers 535 528 528 e550 570 Imports for consumption 322 378 354 313 310 Exports 206 239 220 273 240 Consumption, apparent2 651 668 662 e590 640 Price, average, milled, dollars per metric ton3 186 197 214 226 230 Employment, mine and mill, talc4 239 223 206 208 206 Employment, mine and mill, pyrophyllite4 29 30 31 30 31 Net import reliance5 as a percentage of apparent consumption 22 27 20 7 11 Recycling: Insignificant.