(Parrotia Persica CA Meyer) Leaf Litter on Forest Soil Nutrients
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Status and Protection of Globally Threatened Species in the Caucasus
STATUS AND PROTECTION OF GLOBALLY THREATENED SPECIES IN THE CAUCASUS CEPF Biodiversity Investments in the Caucasus Hotspot 2004-2009 Edited by Nugzar Zazanashvili and David Mallon Tbilisi 2009 The contents of this book do not necessarily reflect the views or policies of CEPF, WWF, or their sponsoring organizations. Neither the CEPF, WWF nor any other entities thereof, assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, product or process disclosed in this book. Citation: Zazanashvili, N. and Mallon, D. (Editors) 2009. Status and Protection of Globally Threatened Species in the Caucasus. Tbilisi: CEPF, WWF. Contour Ltd., 232 pp. ISBN 978-9941-0-2203-6 Design and printing Contour Ltd. 8, Kargareteli st., 0164 Tbilisi, Georgia December 2009 The Critical Ecosystem Partnership Fund (CEPF) is a joint initiative of l’Agence Française de Développement, Conservation International, the Global Environment Facility, the Government of Japan, the MacArthur Foundation and the World Bank. This book shows the effort of the Caucasus NGOs, experts, scientific institutions and governmental agencies for conserving globally threatened species in the Caucasus: CEPF investments in the region made it possible for the first time to carry out simultaneous assessments of species’ populations at national and regional scales, setting up strategies and developing action plans for their survival, as well as implementation of some urgent conservation measures. Contents Foreword 7 Acknowledgments 8 Introduction CEPF Investment in the Caucasus Hotspot A. W. Tordoff, N. Zazanashvili, M. Bitsadze, K. Manvelyan, E. Askerov, V. Krever, S. Kalem, B. Avcioglu, S. Galstyan and R. Mnatsekanov 9 The Caucasus Hotspot N. -

Downloaded from Brill.Com10/08/2021 11:33:23AM Via Free Access 116 IAWA Bulletin N.S., Vol
1AWA Bulletin n.s., Vol. 11 (2), 1990: 115-140 IAWA·IUFRO WOOD ANATOMY SYMPOSIUM 1990 The third Euro-African regional wood anatomy symposium organised by the Wood Science and Technology Laboratories of the ETH (Swiss Federal Institute ofTechnology), Zürich, Switzerland, July 22-27, 1990. Organising Committee Prof. Dr. H.H. Bosshard, Honorary President Dr. L.J. Kucera, Executive Secretary and Local Host Ms. C. Dominquez, Symposium Office Secretary Dr. K. J. M. Bonsen, Deputy Executive Secretary lng. B.J.H. ter Welle, on behalf ofIAWA Prof. Dr. P. Baas, on behalf of IUFRO S 5.01 ABSTRACfS OF PAPERS AND POSTERS C. ANGELACCIO, A. SCffiRONE and B. SCHI MARIAN BABIAK, 1GOR CuNDERLfK and JO RONE, Dipartimento di Scienze deli' Ambiente ZEF KUDELA, Faculty of Wood Technology, Forestale e delle Sue Risorse, Facolta di University of Forestry and Wood Technol Agraria, Universita degli Studi della Tuscia, ogy, Department of Wood Science and Me Via S. Camillo de Lellis, 01100 Viterbo, chanical Wood, 96053 Zvolen, Czechoslo 1taly. - Wood anatomy of Quercus cre· vakia. - Permeability and structure of nata Lam. beech wood. Quercus crenata Lam. (Q. pseudosuber Flow of water and other liquids through G. Santi) is a natural hybrid between Q. cer beech wood (Fagus sylvatica L.) caused by ris x Q. suber. The species is widespread in the external pressure gradient is described by the mediterrane an basin, from France to Al the steady-state Darcy's law. The validity of bania. 1t occurs throughout Italy, usually as the law was proved up to a critical value. The single trees recognisable by their evergreen critical external pressure gradient obtained in and polymorphous leaves; the bark and acorn our experiments was 0.15 MPa/cm. -

A Sibling Species of the Persian Parrotia
The Chinese Parrotia: A Sibling Species of the Persian Parrotia Jianhua Li and Peter Del Tredici he Persian ironwood (Parrotia persica) The Persian and Chinese ironwoods are has a well-deserved reputation as a beau- members of the witch hazel family (Hama- Ttiful garden plant—mainly because of its melidaceae), and in order to appreciate their exfoliating bark and gorgeous fall color—but uniqueness and evolutionary history we need also as a tough species that tolerates drought, to first examine one of their more familiar rela- heat, wind, and cold (Dirr 1998). Less well tives, the witch hazels (Hamamelis). There are known is the fact that Persian ironwood has five species of witch hazel distributed through- a sister species, the Chinese ironwood (Parro- out the temperate regions: H. mollis in eastern tia subaequalis) (Figure 1), growing about 5600 China, H. japonica in Japan, and H. virginiana, kilometers (3500 miles) away in eastern China. H. vernalis, H. mexicana in North America. Remarkably, this species was correctly identi- The genus shows the intercontinental disjunct fied only sixteen years ago (Deng et al. 1992a). distribution between eastern Asia and North Figure 1. Geographic distribution of Parrotia persica (in green) and P. subaequalis (in red). Note that the scale bar is 400 kilometers. Parrotia 3 Hamamelis virginiana ..... Distyliopsis tutcheri 55 84 Distylium racemosum UBC Botanical Garden Sycopsis sinensis ................... 100 ➙ MOBOT Parrotia persica ........... 91 7.8±3.8 mya Parrotia subaequalis ................................. 50 mya Parrotiopsis jacquemontana ......... Fothergilla major .... 10 changes Figure 2. Evolutionary relationships of Hamamelis and petalless genera, showing shift (the arrow) from insect to wind pollination. -

Witch-Hazel - Wikipedia, the Free Encyclopedia
Witch-hazel - Wikipedia, the free encyclopedia http://en.wikipedia.org/wiki/Witch-hazel You can support Wikipedia by making a tax-deductible donation. Witch-hazel From Wikipedia, the free encyclopedia Witch-hazel (Hamamelis) is a genus of flowering plants in the Witch-hazel family Hamamelidaceae, with two species in North America (H. virginiana and H. vernalis), and one each in Japan (H. japonica) and China (H. mollis). They are deciduous shrubs or (rarely) small trees growing to 3-8 m tall, rarely to 12 m tall. The leaves are alternately arranged, oval, 4-16 cm long and 3-11 cm broad, with a smooth or wavy margin. The horticultural name means "together with fruit"; its fruit, flowers, and next year's leaf buds all appear on the branch simultaneously, a rarity among trees. [1] The flowers are sometimes produced on the leafless stems in winter, thus one alternative name for the plant, "Winterbloom". [1] Each flower has four slender strap-shaped petals 1-2 cm long, pale to dark yellow, orange, or red. The fruit is a two-part capsule 1 cm long, containing a single 5 mm glossy black seed in each of the two parts; the capsule splits explosively at maturity in the autumn about 8 months after flowering, ejecting the seeds with sufficient force to fly for distances of up to 10 m, thus another Hamamelis virginiana alternative name "Snapping Hazel". [1] Scientific classification Kingdom: Plantae Hamamelis species are used as food plants by the larvae of Division: Magnoliophyta some Lepidoptera species including Feathered Thorn. Class: Magnoliopsida The name Witch has its origins in Middle English wiche, from Order: Saxifragales the Old English wice, meaning "pliant" or "bendable". -

Integrating Palaeontological and Molecular Data Uncovers Multiple
Integrating palaeontological and molecular data uncovers multiple ancient and recent dispersals in the pantropical Hamamelidaceae Xiaoguo Xiang, Kunli Xiang, Rosa del C. Ortiz, Florian Jabbour, Wei Wang To cite this version: Xiaoguo Xiang, Kunli Xiang, Rosa del C. Ortiz, Florian Jabbour, Wei Wang. Integrating palaeontolog- ical and molecular data uncovers multiple ancient and recent dispersals in the pantropical Hamamel- idaceae. Journal of Biogeography, Wiley, 2019, 46 (11), pp.2622-2631. 10.1111/jbi.13690. hal- 02612865 HAL Id: hal-02612865 https://hal.archives-ouvertes.fr/hal-02612865 Submitted on 19 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Integrating palaeontological and molecular data uncovers multiple ancient and recent dispersals in the pantropical Hamamelidaceae Xiaoguo Xiang1,2, Kunli Xiang1,3, Rosa Del C. Ortiz4, Florian Jabbour5, Wei Wang1,3 1State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing, China 2Jiangxi Province Key Laboratory of Watershed Ecosystem -

New Records of Polypores from Iran, with a Checklist of Polypores for Gilan Province
CZECH MYCOLOGY 68(2): 139–148, SEPTEMBER 27, 2016 (ONLINE VERSION, ISSN 1805-1421) New records of polypores from Iran, with a checklist of polypores for Gilan Province 1 2 MOHAMMAD AMOOPOUR ,MASOOMEH GHOBAD-NEJHAD *, 1 SEYED AKBAR KHODAPARAST 1 Department of Plant Protection, Faculty of Agricultural Sciences, University of Gilan, P.O. Box 41635-1314, Rasht 4188958643, Iran. 2 Department of Biotechnology, Iranian Research Organization for Science and Technology (IROST), P.O. Box 3353-5111, Tehran 3353136846, Iran; [email protected] *corresponding author Amoopour M., Ghobad-Nejhad M., Khodaparast S.A. (2016): New records of polypores from Iran, with a checklist of polypores for Gilan Province. – Czech Mycol. 68(2): 139–148. As a result of a survey of poroid basidiomycetes in Gilan Province, Antrodiella fragrans, Ceriporia aurantiocarnescens, Oligoporus tephroleucus, Polyporus udus,andTyromyces kmetii are newly reported from Iran, and the following seven species are reported as new to this province: Coriolopsis gallica, Fomitiporia punctata, Hapalopilus nidulans, Inonotus cuticularis, Oligo- porus hibernicus, Phylloporia ribis,andPolyporus tuberaster. An updated checklist of polypores for Gilan Province is provided. Altogether, 66 polypores are known from Gilan up to now. Key words: fungi, hyrcanian forests, poroid basidiomycetes. Article history: received 28 July 2016, revised 13 September 2016, accepted 14 September 2016, published online 27 September 2016. Amoopour M., Ghobad-Nejhad M., Khodaparast S.A. (2016): Nové nálezy chorošů pro Írán a checklist chorošů provincie Gilan. – Czech Mycol. 68(2): 139–148. Jako výsledek systematického výzkumu chorošotvarých hub v provincii Gilan jsou publikovány nové druhy pro Írán: Antrodiella fragrans, Ceriporia aurantiocarnescens, Oligoporus tephroleu- cus, Polyporus udus a Tyromyces kmetii. -

Three Further Triterpenoid Saponins from Gleditsia Caspica Fruits and Protective Effect of the Total Saponin Fraction on Cyclophosphamide-Induced Genotoxicity in Mice
Z. Naturforsch. 2015; 70(1-2)c: 31–37 Farouk R. Melek, Fawzia A. Aly, Iman A.A. Kassem*, Mona A.M. Abo-Zeid, Ayman A. Farghaly and Zeinab M. Hassan Three further triterpenoid saponins from Gleditsia caspica fruits and protective effect of the total saponin fraction on cyclophosphamide-induced genotoxicity in mice Abstract: Three triterpenoidal saponins were isolated 1 Introduction from the saponin fraction derived from a Gleditsia caspica Desf. methanolic fruit extract. The isolated saponins were The genus Gleditsia (family Fabaceae) comprises 14 identified as gleditsiosides B, C, and Q based on spectral species of deciduous trees [1]. Gleditsia caspica (Caspian data. The saponin-containing fraction was evaluated in locust), a tree that grows up to 12 m, is cultivated in public vivo for genotoxic and antigenotoxic activities. The frac- gardens in Egypt mainly for ornamental purposes due tion caused no DNA damage in Swiss albino male mice to its graceful habit, elegant form, and delicate fern-like treated with a dose of 45 mg/kg body weight for 24 h, foliage. Gleditsia species have been widely used in folk although it significantly inhibited the number of chromo- medicine. The thorns of G. sinensis have been used for the somal aberrations induced by cyclophosphamide (CP) in treatment of carbuncles, scabies, and suppurate skin dis- bone marrow and germ cells when applied before or after eases, whereas the mature pods and anomalous fruits are CP administration. The inhibitory indices in chromosomal mainly used for treating apoplexy, headache, productive aberrations were 59% and 41% for bone marrow and 48% cough, and asthma. -

Parklane Elementary Global Forest Tree Walk
Parklane Elementary Global Forest Tree Walk LEARNING LANDSCAPES Parklane Elementary Global Forest Tree Walk 2015 Learning Landscapes Site data collected in Summer 2014. Written by: Kat Davidson, Karl Dawson, Angie DiSalvo, Jim Gersbach and Jeremy Grotbo Portland Parks & Recreation Urban Forestry 503-823-TREE [email protected] http://portlandoregon.gov/parks/learninglandscapes Cover photos (from top left to bottom right): 1) Cones and foliage of a monkey puzzle tree. 2) The fall color of a Nothofagus alpina. 3) Cupressus dupreziana in its native range. 4) Students plant and water a young tree. 5) The infl orescence of a Muskogee crape myrtle. 6) Closeup of budding fl owers on a sycoparrotia twig. 7) The brightly-colored fruit of the igiri tree. 8) The fl ower of a Xanthoceras sorbifolium. ver. 1/30/2015 Portland Parks & Recreation 1120 SW Fifth Avenue, Suite 1302 Portland, Oregon 97204 (503) 823-PLAY Commissioner Amanda Fritz www.PortlandParks.org Director Mike Abbaté The Learning Landscapes Program Parklane Elementary School The fi rst planting at the Parklane Elementary Global Forest Learning Landscape was in 1999, and since then, the collection has grown to nearly 80 trees. This tree walk identifi es trees planted as part of the Learning Landscape as well as other interesting specimens at the school. What is a Learning Landscape? A Learning Landscape is a collection of trees planted and cared for at a school by students, volunteers, and Portland Parks & Recreation (PP&R) Urban Forestry staff. Learning Landscapes offer an outdoor educational experience for students, as well as environmental and aesthetic benefi ts to the school and surrounding neighborhood. -

Presenting Parrotia Persica 'Vanessa'
Presenting Parrotia persica ‘Vanessa’ Your SMA 2014 Urban Tree of the Year Manager of Parks for Surrey, British Columbia Owen Croy wrote the Tree of Merit column in City Trees about Parrotia persica ‘Vanessa’ just last spring. He is gratified that this tree sailed on to take the big prize, SMA Urban Tree of the Year. Parrotia persica is most often called simply parrotia or Persian ironwood. Here’s an excerpt of Croy’s column about ‘Vanessa’ parrotia. he Persian ironwood tree is native to the lower mountain slopes Tof northern Iran, and it has been planted widely in cities across Europe and North America for many years. It has great colour in the spring, with glossy, green, red-tipped leaves that later turn a darker green through the summer. Fall colour is spectacular, often with leaves of multiple colours on the tree at the same time: orange, purple, yel- low and green. When older, this tree has flaky grey bark that is very attractive, giving it year-round appeal. The cultivar ‘Vanessa’ emerged from Europe in the 1970s and is now widely cultivated in North American nurseries. ‘Vanessa’ is upright, almost columnar, with branches that arch gracefully outward towards the tip. It is a slow-growing small tree, reaching a height of about 11 metres (36 feet) at maturity. Perhaps because of its slow growth rate, Parrotia ‘Vanessa’ fall color • Photo by Owen Croy it seems that much of the available nursery stock is slightly smaller than would be typical for street tree planting programs. It is hardy in USDA zones 4-8. -

Status and Protection of Globally Threatened Species in the Caucasus
STATUS AND PROTECTION OF GLOBALLY THREATENED SPECIES IN THE CAUCASUS CEPF Biodiversity Investments in the Caucasus Hotspot 2004-2009 Edited by Nugzar Zazanashvili and David Mallon Tbilisi 2009 The contents of this book do not necessarily re ect the views or policies of CEPF, WWF, or their sponsoring organizations. Neither the CEPF, WWF nor any other entities thereof, assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, product or process disclosed in this book. Citation: Zazanashvili, N. and Mallon, D. (Editors) 2009. Status and Protection of Globally Threatened Species in the Caucasus. Tbilisi: CEPF, WWF. Contour Ltd., 232 pp. ISBN 978-9941-0-2203-6 Design and printing Contour Ltd. 8, Kargareteli st., 0164 Tbilisi, Georgia December 2009 The Critical Ecosystem Partnership Fund (CEPF) is a joint initiative of l’Agence Française de Développement, Conservation International, the Global Environment Facility, the Government of Japan, the MacArthur Foundation and the World Bank. This book shows the effort of the Caucasus NGOs, experts, scienti c institutions and governmental agencies for conserving globally threatened species in the Caucasus: CEPF investments in the region made it possible for the rst time to carry out simultaneous assessments of species’ populations at national and regional scales, setting up strategies and developing action plans for their survival, as well as implementation of some urgent conservation measures. Contents Foreword 7 Acknowledgments 8 Introduction CEPF Investment in the Caucasus Hotspot A. W. Tordoff, N. Zazanashvili, M. Bitsadze, K. Manvelyan, E. Askerov, V. Krever, S. Kalem, B. Avcioglu, S. Galstyan and R. Mnatsekanov 9 The Caucasus Hotspot N. -

Lepidoptera: Tortricidae) 677-686 Download
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Linzer biologische Beiträge Jahr/Year: 2017 Band/Volume: 0049_1 Autor(en)/Author(s): Maharramova Sheyda Mamed, Ayberk Hamit Artikel/Article: New records of leafrollers reared in Azerbaijan (Lepidoptera: Tortricidae) 677-686 download www.zobodat.at Linzer biol. Beitr. 49/1 677-686 28.7.2017 New records of leafrollers reared in Azerbaijan (Lepidoptera: Tortricidae) Sheyda MAHARRAMOVA & Hamit AYBERK A b s t r a c t : 17 species of tortricid moths had been defined for the eastern parts of Azerbaijan during the years of 1994-2015. Five of these, Ptycholoma lecheana (LINNAEUS, 1758), Cacoecimorpha pronubana HÜBNER, 1799, Eudemis profundana ([DENIS & SCHIFFERMÜLLER], 1775), Hedya salicella (LINNAEUS, 1758), and Epinotia demarniana (FISCHER VON RÖSLERSTAMM, 1840), are new to the Azerbaijan fauna; two of them (Cacoecimorpha pronubana and Epinotia demarniana) are new to the Caucasian fauna, as well. Leafrollers were collected in the larval and pupal stage from March to September on their food plants according to the sampling methods. The early stages were kept in the laboratory until adult emergence, after which they were killed and pinned. Data on newly recorded leafrollers are given in the text including species name, collection area, coordinates of area, data of collection, food plants on which they were recorded, and sex of specimen(s). Key words: Tortricids, damage, host plants, Azerbaijan, fauna. Introduction Tortricidae, commonly known as leafrollers, are one of the most diverse families in the Microlepidoptera. The number of leafroller species in countries bordering Azerbaijan differs among the adjacent regions: 469 species are recorded from Turkey (KOÇAK & KEMAL 2012), 145 species from Iran (KOÇAK & KEMAL 2012), and 139 species from Georgia (ESARTIA 1988). -
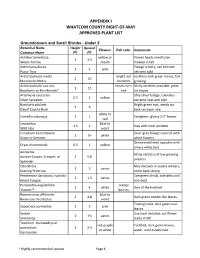
Whatcom County's Approved Plant List
APPENDIX I WHATCOM COUNTY RIGHT-OF-WAY APPROVED PLANT LIST Groundcovers and Small Shrubs - Under 2' Botanical Name Height Spread Flowers Fall color Comments Common Name (ft) (ft) Achillea tomentosa yellow or Flower heads need to be 1 3-5 Wooly Yarrow cream mowed in fall Antennaria dioica Foliage is furry, can tolerate 1 2 pink Pussy Toes extreme cold Arctostaphylos media bright red Leathery dark green leaves, fast 2 10 Manzanita Media branches growing Arctostaphylos uva ursi leaves turn Many varieties available, great 1 15 Bearberry or Kinnikinnick* red for slopes Artemesia caucasica Silky silver foliage, tolerates 0.5 2 yellow Silver Spreader extreme heat and cold Baccharis pilularis Bright green mat, needs cut 2 6 Dwarf Coyote Bush back once per year white to Camellia sasanqua 1 1 Evergreen, glossy 2-3" leaves red Ceanothus blue to 1.5 2 Stay with local varieties Wild Lilac violet Cerastium tomentosum Silver gray foliage covered with 1 5+ white Snow-in-Summer white flowers Ornamental seed capsules with Dryas drummondii 0.5 1 yellow silvery white tails Juniperus Many varieties of low growing Juniper-Carpet, Creeper, or 2 5-8 junipers Spreader Oenothera May die back in severe winters, 1 2 varies Evening Primrose come back strong Penstemon davidsonii, rupicola Evergreen shrub, tolerates cold 2 1.5 varies Beard Tongue and heat Pyracantha augustifolia orange 1 4 white One of the hardiest 'Gnome'* berries Rosmariunus officinalis blue to 2 4-8 Dark green needle-like leaves Rosemary 'Prostratus' violet Trailing habit, dark green oval Saponaria ocymoides 1 3 pink leaves Sedum Use local varieties, cut flower 2 1-5 varies Stonecrop stalks in fall Teucrium chamaedrys or red-purple Toothed, dark green leaves, postratium 1 2-3 or white water until established Germander * Highly-recommended species Page 1 Thymus white to 2 1-2 Many varieties Thyme purple Shrubs Botanical Name Height Spread Flowers Fall color Comments Common Name (ft) (ft) Arctostaphylos bright red Shiny dark green leaves turn 2-15 20 varies Manzanita berries maroon in fall.