Oral Pharmaceutical Solutions Comrising Nortriptyline Hydrochloride
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Registration Certificate
1 The following information has been supplied by the Greek Aliens Bureau: It is obligatory for all EU nationals to apply for a “Registration Certificate” (Veveosi Engrafis - Βεβαίωση Εγγραφής) after they have spent 3 months in Greece (Directive 2004/38/EC).This requirement also applies to UK nationals during the transition period. This certificate is open- dated. You only need to renew it if your circumstances change e.g. if you had registered as unemployed and you have now found employment. Below we outline some of the required documents for the most common cases. Please refer to the local Police Authorities for information on the regulations for freelancers, domestic employment and students. You should submit your application and required documents at your local Aliens Police (Tmima Allodapon – Τμήμα Αλλοδαπών, for addresses, contact telephone and opening hours see end); if you live outside Athens go to the local police station closest to your residence. In all cases, original documents and photocopies are required. You should approach the Greek Authorities for detailed information on the documents required or further clarification. Please note that some authorities work by appointment and will request that you book an appointment in advance. Required documents in the case of a working person: 1. Valid passport. 2. Two (2) photos. 3. Applicant’s proof of address [a document containing both the applicant’s name and address e.g. photocopy of the house lease, public utility bill (DEH, OTE, EYDAP) or statement from Tax Office (Tax Return)]. If unavailable please see the requirements for hospitality. 4. Photocopy of employment contract. -

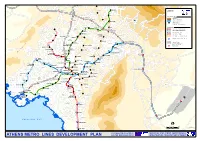
Athens Metro Lines Development Plan and the European Union Transport and Networks
Kifissia M t . P e Zefyrion Lykovrysi KIFISSIA n t LEGEND e l i Metamorfosi KAT METRO LINES NETWORK Operating Lines Pefki Nea Penteli LINE 1 Melissia PEFKI LINE 2 Kamatero MAROUSSI LINE 3 Iraklio Extensions IRAKLIO Penteli LINE 3, UNDER CONSTRUCTION NERANTZIOTISSA OTE AG.NIKOLAOS Nea LINE 2, UNDER DESIGN Filadelfia NEA LINE 4, UNDER DESIGN IONIA Maroussi IRINI PARADISSOS Petroupoli Parking Facility - Attiko Metro Ilion PEFKAKIA Nea Vrilissia Ionia ILION Aghioi OLYMPIAKO "®P Operating Parking Facility STADIO Anargyri "®P Scheduled Parking Facility PERISSOS Nea PALATIANI Halkidona SUBURBAN RAILWAY NETWORK SIDERA Suburban Railway DOUK.PLAKENTIAS Anthousa ANO Gerakas PATISSIA Filothei "®P Suburban Railway Section also used by Metro o Halandri "®P e AGHIOS HALANDRI l P "® ELEFTHERIOS ALSOS VEIKOU Kallitechnoupoli a ANTHOUPOLI Galatsi g FILOTHEI AGHIA E KATO PARASKEVI PERISTERI GALATSI Aghia . PATISSIA Peristeri P Paraskevi t Haidari Psyhiko "® M AGHIOS NOMISMATOKOPIO AGHIOS Pallini ANTONIOS NIKOLAOS Neo PALLINI Pikermi Psihiko HOLARGOS KYPSELI FAROS SEPOLIA ETHNIKI AGHIA AMYNA P ATTIKI "® MARINA "®P Holargos DIKASTIRIA Aghia PANORMOU ®P KATEHAKI Varvara " EGALEO ST.LARISSIS VICTORIA ATHENS ®P AGHIA ALEXANDRAS " VARVARA "®P ELEONAS AMBELOKIPI Papagou Egaleo METAXOURGHIO OMONIA EXARHIA Korydallos Glyka PEANIA-KANTZA AKADEMIA GOUDI Nera "®P PANEPISTIMIO MEGARO MONASTIRAKI KOLONAKI MOUSSIKIS KORYDALLOS KERAMIKOS THISSIO EVANGELISMOS ZOGRAFOU Nikea SYNTAGMA ANO ILISSIA Aghios PAGRATI KESSARIANI Ioannis ACROPOLI NEAR EAST Rentis PETRALONA NIKEA Tavros Keratsini Kessariani SYGROU-FIX KALITHEA TAVROS "®P NEOS VYRONAS MANIATIKA Spata KOSMOS Pireaus AGHIOS Vyronas s MOSCHATO Peania IOANNIS o Dafni t Moschato Ymittos Kallithea ANO t Drapetsona i PIRAEUS DAFNI ILIOUPOLI FALIRO Nea m o Smyrni Y o Î AGHIOS Ilioupoli DIMOTIKO DIMITRIOS . -

Supplementary Materials
Supplementary Materials Figure S1. Temperature‐mortality association by sector, using the E‐OBS data. Municipality ES (95% CI) CENTER Athens 2.95 (2.36, 3.54) Subtotal (I-squared = .%, p = .) 2.95 (2.36, 3.54) . EAST Dafni-Ymittos 0.56 (-1.74, 2.91) Ilioupoli 1.42 (-0.23, 3.09) Kessariani 2.91 (0.39, 5.50) Vyronas 1.22 (-0.58, 3.05) Zografos 2.07 (0.24, 3.94) Subtotal (I-squared = 0.0%, p = 0.689) 1.57 (0.69, 2.45) . NORTH Aghia Paraskevi 0.63 (-1.55, 2.87) Chalandri 0.87 (-0.89, 2.67) Galatsi 1.71 (-0.57, 4.05) Gerakas 0.22 (-4.07, 4.70) Iraklio 0.32 (-2.15, 2.86) Kifissia 1.13 (-0.78, 3.08) Lykovrisi-Pefki 0.11 (-3.24, 3.59) Marousi 1.73 (-0.30, 3.81) Metamorfosi -0.07 (-2.97, 2.91) Nea Ionia 2.58 (0.66, 4.54) Papagos-Cholargos 1.72 (-0.36, 3.85) Penteli 1.04 (-1.96, 4.12) Philothei-Psychiko 1.59 (-0.98, 4.22) Vrilissia 0.60 (-2.42, 3.71) Subtotal (I-squared = 0.0%, p = 0.975) 1.20 (0.57, 1.84) . PIRAEUS Aghia Varvara 0.85 (-2.15, 3.94) Keratsini-Drapetsona 3.30 (1.66, 4.97) Korydallos 2.07 (-0.01, 4.20) Moschato-Tavros 1.47 (-1.14, 4.14) Nikea-Aghios Ioannis Rentis 1.88 (0.39, 3.39) Perama 0.48 (-2.43, 3.47) Piraeus 2.60 (1.50, 3.71) Subtotal (I-squared = 0.0%, p = 0.580) 2.25 (1.58, 2.92) . -

Villa Karissa
LuxuryVilla Villa inKarissa Zakynthos Greece Welcome Set amongst the olive groves in the tiny village of GERAKAS, to the south of the island of ZAKYNTHOS ZANTE, GREECE, is the three bedroom stone built Villa Karissa. It is available for holiday rental from May to October from just £520 per week. A five five minute walk to local shops, tavernas, the turtle research centre and the delightful Gerakas secluded and sandy beach. The town of Zante and its fishing port is just a 30 minute drive away It is the perfect place for a relaxing & peaceful holiday, exclusively yours for the duration of your stay. We are not a package holiday company, this is our own private villa, so you have to book your own flights from whatever airport you prefer and at times that suit you. Whilst we leave you alone while you're on holiday in our villa you're not totally abandoned as we do have our representatives to sort out any problems should they occur and to advise on excursions, car hire and local amenities should you need them to The villa itself is 148sqm and the surrounding private garden is 1500sqm. The freshwater swimming pool is 8m x 3.5m and because the villa is south facing it captures the sunlight from dawn to dusk. Each room has its own superb view of either olive groves, mountain area or the Ionian Sea with views of surrounding Greek Islands. Boat trips and excursions are available locally to tour the island of Zante and ferry's to take you to Kafelonia or the Peloponnese Islands. -
Athens Metro Lines Development Plan and the European Union Infrastructure, Transport and Networks
AHARNAE Kifissia M t . P ANO Lykovrysi KIFISSIA e LIOSIA Zefyrion n t LEGEND e l i Metamorfosi KAT OPERATING LINES METAMORFOSI Pefki Nea Penteli LINE 1, ISAP IRAKLIO Melissia LINE 2, ATTIKO METRO LIKOTRIPA LINE 3, ATTIKO METRO Kamatero MAROUSSI METRO STATION Iraklio FUTURE METRO STATION, ISAP Penteli IRAKLIO NERATZIOTISSA OTE EXTENSIONS Nea Filadelfia LINE 2, UNDER CONSTRUCTION KIFISSIAS NEA Maroussi LINE 3, UNDER CONSTRUCTION IRINI PARADISSOS Petroupoli IONIA LINE 3, TENDERED OUT Ilion PEFKAKIA Nea Vrilissia LINE 2, UNDER DESIGN Ionia Aghioi OLYMPIAKO PENTELIS LINE 4, UNDER DESIGN & TENDERING AG.ANARGIRI Anargyri STADIO PERISSOS Nea "®P PARKING FACILITY - ATTIKO METRO Halkidona SIDERA DOUK.PLAKENTIAS Anthousa Suburban Railway Kallitechnoupoli ANO Gerakas PATISSIA Filothei Halandri "®P o ®P Suburban Railway Section " Also Used By Attiko Metro e AGHIOS HALANDRI l "®P ELEFTHERIOS ALSOS VEIKOU Railway Station a ANTHOUPOLI Galatsi g FILOTHEI AGHIA E KATO PARASKEVI PERISTERI . PATISSIA GALATSI Aghia Peristeri THIMARAKIA P Paraskevi t Haidari Psyhiko "® M AGHIOS NOMISMATOKOPIO AGHIOS Pallini NIKOLAOS ANTONIOS Neo PALLINI Pikermi Psihiko HOLARGOS KYPSELI FAROS SEPOLIA ETHNIKI AGHIA AMYNA P ATTIKI "® MARINA "®P Holargos DIKASTIRIA Aghia PANORMOU ®P ATHENS KATEHAKI Varvara " EGALEO ST.LARISSIS VICTORIA ATHENS ®P AGHIA ALEXANDRAS " VARVARA "®P ELEONAS AMBELOKIPI Papagou Egaleo METAXOURGHIO OMONIA EXARHIA Korydallos Glyka PEANIA-KANTZA AKADEMIA GOUDI Nera PANEPISTIMIO KERAMIKOS "®P MEGARO MONASTIRAKI KOLONAKI MOUSSIKIS KORYDALLOS ZOGRAFOU THISSIO EVANGELISMOS Zografou Nikea ROUF SYNTAGMA ANO ILISSIA Aghios KESSARIANI PAGRATI Ioannis ACROPOLI Rentis PETRALONA NIKEA Tavros Keratsini Kessariani RENTIS SYGROU-FIX P KALITHEA TAVROS "® NEOS VYRONAS MANIATIKA Spata KOSMOS LEFKA Pireaus AGHIOS Vyronas s MOSHATO IOANNIS o Peania Dafni t KAMINIA Moshato Ymittos Kallithea t Drapetsona PIRAEUS DAFNI i FALIRO Nea m o Smyrni Y o Î AGHIOS Ilioupoli DIMOTIKO DIMITRIOS . -

Implementation of Noise Barriers in Attiki Odos Motorway Based on the Relevant Strategic Noise Mapping and Noise Action Plan
Implementation of Noise Barriers in Attiki Odos Motorway based on the relevant Strategic Noise Mapping and Noise Action Plan Konstantinos Vogiatzis Associate Professor, Laboratory of Transportation Environmental Acoustics (L.T.E.A.), Faculty of the Civil Engineering, University of Thessaly, Volos, Greece Vassiliki Zafiropoulou Dr. Researcher Laboratory of Transportation Environmental Acoustics (L.T.E.A.), Faculty of the Civil Engineering, University of Thessaly, Volos, Greece Summary Attiki Odos Motorway (the “Athens ring road”) constitutes one of the most important co-financed road projects in Europe, laying the foundations for the execution of a series of successful road concession projects in Greece. As per the Environmental Noise Protection and Monitoring, Attiki Odos has already implemented in 2009-2010 a Strategic Noise Map (SNM) and Noise Action Plan, followed by a full update in 2014 according to the directive 2002/49/EC (END). The updated Noise Action Plan (NAP), foreseen as main actions against road traffic noise, the implementation of reflective transparent noise barriers, partial motorway covers and also urban design tools. In order to validate the results, a comparison of the calculated noise indices Lden & Lnight results with the measured noise levels in more than 150 locations along the motorway - from the annual environmental noise monitoring program - was implemented proving exceptional correlation. According to the most recent results of the noise monitoring program of Attiki Odos during 2017, in the Geographical Section A13 (from K.P.32+600 to K.P.32+980 in both directions), a significant exceedance of levels of the above noise indicators, was documented as well as important residents’ complaints. -

Αthens and Attica in Prehistory Proceedings of the International Conference Athens, 27-31 May 2015
Αthens and Attica in Prehistory Proceedings of the International Conference Athens, 27-31 May 2015 edited by Nikolas Papadimitriou James C. Wright Sylvian Fachard Naya Polychronakou-Sgouritsa Eleni Andrikou Archaeopress Archaeology Archaeopress Publishing Ltd Summertown Pavilion 18-24 Middle Way Summertown Oxford OX2 7LG www.archaeopress.com ISBN 978-1-78969-671-4 ISBN 978-1-78969-672-1 (ePdf) © 2020 Archaeopress Publishing, Oxford, UK Language editing: Anastasia Lampropoulou Layout: Nasi Anagnostopoulou/Grafi & Chroma Cover: Bend, Nasi Anagnostopoulou/Grafi & Chroma (layout) Maps I-IV, GIS and Layout: Sylvian Fachard & Evan Levine (with the collaboration of Elli Konstantina Portelanou, Ephorate of Antiquities of East Attica) Cover image: Detail of a relief ivory plaque from the large Mycenaean chamber tomb of Spata. National Archaeological Museum, Athens, Department of Collection of Prehistoric, Egyptian, Cypriot and Near Eastern Antiquities, no. Π 2046. © Hellenic Ministry of Culture and Sports, Archaeological Receipts Fund All rights reserved. No part of this publication may be reproduced or transmitted, in any form or by any means, electronic, mechanical, photocopying, or otherwise, without the prior permission of the publisher. Printed in the Netherlands by Printforce This book is available direct from Archaeopress or from our website www.archaeopress.com Publication Sponsors Institute for Aegean Prehistory The American School of Classical Studies at Athens The J.F. Costopoulos Foundation Conference Organized by The American School of Classical Studies at Athens National and Kapodistrian University of Athens - Department of Archaeology and History of Art Museum of Cycladic Art – N.P. Goulandris Foundation Hellenic Ministry of Culture and Sports - Ephorate of Antiquities of East Attica Conference venues National and Kapodistrian University of Athens (opening ceremony) Cotsen Hall, American School of Classical Studies at Athens (presentations) Museum of Cycladic Art (poster session) Organizing Committee* Professor James C. -

The Christianization of the Peloponnese
THE CHRISTIANIZATION OF THE Antique churches in the Peloponnese PELOPONNESE: THE CASE FOR STRATEGIC undertaken in 2012, allows a synthetic CHANGE interpretation of all the material within the surrounding landscape to be possible.i While ABSTRACT the precise chronologies may remain elusive, The issue of the persistence of paganism is this present study shows how sociological now quite well considered; however, it is only theories of conversion processes can be in recent times that the same concern, applied to the topographic analysis of the late approached from another perspective, the antique churches of the Peloponnese to help multifaceted nature of the Christianization of determine the nature of Christianization the Peloponnese, has become the topic of across the diachronic range. In this work I will detailed discussion. It is likely that present some new theories regarding Christianization in Achaia took place processes and phases of conversion, and the incrementally and with a variety of effects implications of these in terms of according to the location (Sweetman 2010). understanding networks and society in the The processes of how this took place and Late Antique Peloponnese. under what circumstances remain to be discussed in detail. As a considered and active INTRODUCTIONii process, understanding methods of Epigraphic evidence indicates a steady growth conversion should provide insights into the οf a Christian presence in the Peloponnese nature of society at the time, particularly in throughout the 4th century (Foschia 2009, terms of communications. Church location 209-33), but the monumentalization of reflects a range of choices made in terms of Christianity here is comparatively late. -

Econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Sidiropoulos, E. Conference Paper The impact of the new airport of Athens on the land values of Eastern Attica 38th Congress of the European Regional Science Association: "Europe Quo Vadis? - Regional Questions at the Turn of the Century", 28 August - 1 September 1998, Vienna, Austria Provided in Cooperation with: European Regional Science Association (ERSA) Suggested Citation: Sidiropoulos, E. (1998) : The impact of the new airport of Athens on the land values of Eastern Attica, 38th Congress of the European Regional Science Association: "Europe Quo Vadis? - Regional Questions at the Turn of the Century", 28 August - 1 September 1998, Vienna, Austria, European Regional Science Association (ERSA), Louvain-la-Neuve This Version is available at: http://hdl.handle.net/10419/113397 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. -

ΟΔΗΓΙΕΣ ΠΡΟΣΒΑΣΗΣ Στα Ελληνικά DIRECTIONS in English
ΟΔΗΓΙΕΣ ΠΡΟΣΒΑΣΗΣ Στα Ελληνικά DIRECTIONS In English ΟΔΗΓΙΕΣ ΠΡΟΣΒΑΣΗΣ Η Διεύθυνση της Εταιρείας είναι η ακόλουθη: Micrel Ιατρικά Μηχανήματα ΑΕ Οδός Γέρακα 113 , 15344 Γέρακας, Αθήνα, Ελλάδα Τηλ: +30 210 6032333 | +30 210 6032334 Φάξ: +30 210 6032335 E-mail: [email protected] Με Αυτοκίνητο / Taxi: . Κατεύθυνση από Ραφήνα προς Αθήνα, επί της Λεωφόρου Μαραθώνος. (Κοντινότερη έξοδος Αττικής Οδού: ‘Έξοδος 15 – Λεωφόρος Μαραθώνος) Οδηγείτε επί της Λεωφόρου Μαραθώνος και ακριβώς μετά το σουπερμάρκετ Carrefour στρίβετε δεξιά στην οδό Έβρου. Συνεχίζετε ευθεία στην οδό Έβρου η οποία οδηγεί στη συνέχεια στην Οδό Γέρακα. Η Micrel βρίσκετε στο αριστερό σας χέρι, περίπου 5’ λεπτά οδήγησης από τη Λεωφόρο Μαραθώνος. Google maps: http://goo.gl/maps/L7Egl . Κατεύθυνση από Αθήνα προς Ραφήνα, επί της Λεωφόρου Μαραθώνος. Οδηγείτε επί της Λεωφόρου Μαραθώνος και ακριβώς πριν το σουπερμάρκετ Carrefour στρίβετε αριστερά στην οδό Έβρου. Συνεχίζετε ευθεία στην οδό Έβρου η οποία οδηγεί στη συνέχεια στην Οδό Γέρακα. Η Micrel βρίσκετε στο αριστερό σας χέρι, περίπου 5’ λεπτά οδήγησης από τη Λεωφόρο Μαραθώνος. Google maps: http://goo.gl/maps/GIsWF Με Μέσα Μαζικής Μεταφοράς: . Ο κοντινότερος σταθμός του Μετρό είναι ο Σταθμός Δουκ. Πλακεντίας (Μπλε Γραμμή). http://www.ametro.gr/files/images/AM_Athens_Metro_map_April_2013_gr.jpg http://www.ametro.gr/page/default.asp?id=48&la=1# Από τον σταθμό του Μετρό για να έρθετε στην εταιρεία, μπορείτε να χρησιμοποιήσετε το Λεωφορείο 302 ΣΤΑΘΜΟΣ ΔΟΥΚ. ΠΛΑΚΕΝΤΙΑΣ - ΓΕΡΑΚΑ (ΚΥΚΛΙΚΗ) και να κατέβετε στην Στάση NIVEA. Το λεωφορείο σας αφήνει επί της οδού Γέρακα πολύ κοντά στην εταιρεία, η οποία βρίσκεται στον αριθμό 113. Πρόγραμμα Δρομολογίων Λεωφορείου 302. http://www.oasa.gr/xmap.php?id=p302 Επίσης, κοντά στην εταιρεία βρίσκεται ο σταθμός του Προαστιακού «ΠΑΛΛΗΝΗ» αλλά δεν υπάρχει απευθείας γραμμή λεωφορείου προς την εταιρεία και κατά συνέπεια απαιτείται η χρήση Ταξί. -

Social and Spatial Dimensions of Homelessness in Athens
Title: Social and Spatial Dimensions of Homelessness in Athens: Welfare Networks and Practices of Care Professionals By Vassilios Petrou Arapoglou The London School of Economics and Political Science 2002 Submitted for the degree of Doctor of Philosophy (PhD) UMI Number: U171992 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. Dissertation Publishing UMI U171992 Published by ProQuest LLC 2014. Copyright in the Dissertation held by the Author. Microform Edition © ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 POLITICAL f44£:S£-S F 961+73,0 1 ACKNOWLEDGEMENTS Acknowledgements go to my friends, family, and teachers for their support and advice. I would also like to extend my appreciation to all the people who gave so much of their time in interviews and to the homeless people who talked to me about some of their experiences in shelters and in institutions. To my friend Dimitris Christopoulos I owe the greatest debt because he has constantly provided support from the very beginning to the very end of the thesis and he encouraged me to continue in many difficult moments of this endeavour. Thanks go to my former colleagues in the ‘actual workplace* and particularly, to those who contributed to this research by sharing their information, linkages, and expertise: Sofia, Maria, Angeliki. -

18-05-21 Acts Against Religious Sites in Greece
MINISTRY OF EDUCATION AND RELIGIOUS AFFAIRS GENERAL SECRETARIAT FOR RELIGIOUS AFFAIRS DIRECTORATE FOR RELIGIOUS EDUCATION AND INTERFAITH RELATIONS DEPARTMENT FOR RELIGIOUS FREEDOMS AND INTERFAITH RELATIONS ACTS AGAINST RELIGIOUS SITES IN GREECE REPORT 2019 All maps and statistical analysis are also available, in Greek and in English, on the website: https://storymaps.arcgis.com/stories/458d92cb4558471bb59b78f2597b596c Cover page: Geographical display of all incidents presented in the 2019 Report. Original map-layout source: Hellenic Statistical Authority Back cover: Detail of inscription, Holy Monastery of St. George Hozeva, Israel All maps (except for II.A.2.c.iii) as well as statistical processing and data presentation were prepared, upon request and instructions by the General Secretariat for Religious Affairs, by V. Rev. Archimandrite Ioannis-Georgios Peristerides, Chemical Engineer MSc, Theologian MA, PhD candidate of School of Rural and Surveying Engineering, National Technical University of Athens- whom we warmly thank - with the support of the research group “GeoCHOROS” of the National Technical University of Athens and were granted free of charge. Copyright ©2020 General Secretariat for Religious Affairs, Ministry of Education and Religious Affairs This work is licensed under the Creative Commons Attribution 4.0 International License http://creativecommons.org/licenses/by/4.0/ The Report (in Greek and in English) is available online on the website of the Ministry of Education and Religious Affairs: www.minedu.gov.gr COURTESY AND NON-OFFICIAL TRANSLATION Content enriched Translated and adapted by Callis MITRAKA 2 “I have loved, O Lord, the beauty of thy house; and the place where thy glory dwelleth” (Psalm, 25:8) . ָשְְ ֵּבב הן ָכ תשמ ,ם ָןְְּו ; ֶָךה ֵּיב ָןעְמ ,י תי ְָ הַה--הָיהְי ( ים הִּ הְִּת ח) “Domine dilexi decorem domus tuae et locum habitationis gloriae tuae” (Ps.