Proquest Dissertations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -

EICG-Hot Spots: EICG Appendix C
[Note: The pre-existing regulation text is set forth below in normal type. The proposed amendments are shown in underline to indicate additions and strikeout to indicate deletions. The square brackets “[ ]” are used to indicate minor adjustments to text (e.g., page numbers and adoption dates) that will be updated upon adoption of the proposed amendments.] APPENDIX C FACILITY GUIDELINE INDEX (FACILITY "LOOK-UP" TABLE) This Page Intentionally Left Blank APPENDIX C - I RESPONSIBILITIES OF ALL FACILITIES NOTES FOR APPENDIX CFACILITY GUIDELINE INDEX APPENDIX C‑I RESPONSIBILITIES OF ALL FACILITIES NOTHING IN THIS APPENDIX SHALL BE CONSTRUED AS REQUIRING THAT SOURCE TESTING BE CONDUCTED FOR SUBSTANCES SET FORTH IN THIS APPENDIX. FURTHER, IN CASES WHERE A SUBSTANCE SET FORTH HEREIN IS NOT PRESENT AT A PARTICULAR FACILITY, THE FACILITY OPERATOR SHALL NOT ATTEMPT TO QUANTIFY THE EMISSIONS OF SUCH SUBSTANCE, BUT SHALL PROVIDE ADEQUATE DOCUMENTATION TO DEMONSTRATE TO THE DISTRICT THAT THE POSSIBLE PRESENCE OF THE SUBSTANCE AT THE FACILITY HAS BEEN ADDRESSED AND THAT THERE ARE NO EMISSIONS OF THE SUBSTANCE FOR SPECIFIED REASONS. Substances emitted by a particular device or process may not be limited to those listed in this Facility Guideline Index. THIS APPENDIX IS NOT AN EXHAUSTIVE LIST. ALL FACILITIES ARE RESPONSIBLE FOR IDENTIFYING AND ACCOUNTING FOR ANY LISTED SUBSTANCE USED, MANUFACTURED, FORMULATED, OR RELEASED. This Facility Guideline Index is arranged in alphabetical order. The first part of the index, Appendix C‑I, lists devices common to many industries and the second part of the index, Appendix C‑II, lists industry types. Extensive cross-referencing has been incorporated into the index, particularly in Appendix C‑II, to identify industries and processes known by alternative names. -

Gas Conversion Factor for 300 Series
300GasTable Rec # Gas Symbol GCF Density (g/L) Density (g/L) 25° C / 1 atm 0° C / 1 atm 1 Acetic Acid C2H4F2 0.4155 2.7 2.947 2 Acetic Anhydride C4H6O3 0.258 4.173 4.555 3 Acetone C3H6O 0.3556 2.374 2.591 4 Acetonitryl C2H3N 0.5178 1.678 1.832 5 Acetylene C2H2 0.6255 1.064 1.162 6 Air Air 1.0015 1.185 1.293 7 Allene C3H4 0.4514 1.638 1.787 8 Ammonia NH3 0.7807 0.696 0.76 9 Argon Ar 1.4047 1.633 1.782 10 Arsine AsH3 0.7592 3.186 3.478 11 Benzene C6H6 0.3057 3.193 3.485 12 Boron Trichloride BCl3 0.4421 4.789 5.228 13 Boron Triflouride BF3 0.5431 2.772 3.025 14 Bromine Br2 0.8007 6.532 7.13 15 Bromochlorodifluoromethane CBrClF2 0.3684 6.759 7.378 16 Bromodifluoromethane CHBrF2 0.4644 5.351 5.841 17 Bromotrifluormethane CBrF3 0.3943 6.087 6.644 18 Butane C4H10 0.2622 2.376 2.593 19 Butanol C4H10O 0.2406 3.03 3.307 20 Butene C4H8 0.3056 2.293 2.503 21 Carbon Dioxide CO2 0.7526 1.799 1.964 22 Carbon Disulfide CS2 0.616 3.112 3.397 23 Carbon Monoxide CO 1.0012 1.145 1.25 24 Carbon Tetrachloride CCl4 0.3333 6.287 6.863 25 Carbonyl Sulfide COS 0.668 2.456 2.68 26 Chlorine Cl2 0.8451 2.898 3.163 27 Chlorine Trifluoride ClF3 0.4496 3.779 4.125 28 Chlorobenzene C6H5Cl 0.2614 4.601 5.022 29 Chlorodifluoroethane C2H3ClF2 0.3216 4.108 4.484 30 Chloroform CHCl3 0.4192 4.879 5.326 31 Chloropentafluoroethane C2ClF5 0.2437 6.314 6.892 32 Chloropropane C3H7Cl 0.308 3.21 3.504 33 Cisbutene C4H8 0.3004 2.293 2.503 34 Cyanogen C2N2 0.4924 2.127 2.322 35 Cyanogen Chloride ClCN 0.6486 2.513 2.743 36 Cyclobutane C4H8 0.3562 2.293 2.503 37 Cyclopropane C3H6 0.4562 -

Experimental Deformation of Polyphase Rock Analogues
GEOLOGICA ULTRAJECTINA Mededelingen van de Faculteit Aardwetenschappen der Universiteit Utrecht No. 110 Experimental deformation of polyphase rock analogues PAUL DIRK BONS Experinlelltal defornlation of polyphase rock analogues Experimentele deformatie van polyfase gesteente-analogen (met een samenvatting in het Nederlands) PROEFSCHRIFT TER VERKRllGING VAN DE GRAAD VAN DOCTOR AAN DE UNIVERSITEIT UTRECHT OP GEZAG VAN DE RECTOR MAGNIFICUS, PROF. DR l.A. VAN GINKEL, INGEVOLGE HET BESLUIT VAN HET COLLEGE VAN DECANEN IN HET OPENBAAR TE VERDEDIGEN OP WOENSDAG 22 SEPTEMBER 1993 DES OCHTENDS TE 10.30 UUR DOOR PAUL DIRK BONS GEBOREN OP 20 FEBRUARI 1964 TE SYDNEY, AUSTRALIE PROMOTOREN: PROF. DR S.H. WHITE (FACULTEIT AARDWETENSCHAPPEN, UNIVERSITEIT UTRECHT) PROF. DR C.W. PASSCHIER (lNSTITUT FUR GEOWISSENSCHAFTEN, JOHANNES GUTENBERG-UNIVERSITAT, MAINZ, BONDSREPUBLIEK DUITSLAND) CO-PROMOTOREN: DR J.L. URAl (SHELL RESEARCH B.V., RIJSWIJK) DR M.W. JESSELL (DEPARTMENT OF EARTH SCIENCES, MONASH UNIVERSITY, CLAYTON, AUSTRALIE) Dit proefschrift werd mogelijk gemaakt met financiele steun van de Nederlandse Organisatie voor Wetenschappelijk Onderzoek (N.W.O.), c.q. de Stichting Aardwetenschappelijk Onderzoek Nederland (A.W.O.N.), projectnummer 751-353 021 CIP-GEGEVENS KONINKLIJKE BIBLIOTHEEK, DEN HAAG Bons, Paul Dirk Experimental deformation of polyphase rock analogues / Paul Dirk Bons. - Utrecht: Faculteit Aardwetenschappen der Universiteit Utrecht. (Geologica Ultraiectina, ISSN 0072-1026; no. 110) Proefschrift Universiteit Utrecht. - Met lit. opg. - Met samenvatting in het Nederlands. ISBN 90-71577-64-3. Trefw.: deformatie / polyfase materialen / gesteente-analogen. Some questions on polyphase materials: How many strawberries, how large in the "charlotte auxfraises"? How to characterise their distribution? How should one put the pears (aspect ratio, distribution .. -

And Perfluoroalkyl Substances (PFAS): Sources, Pathways and Environmental Data
Poly- and perfluoroalkyl substances (PFAS): sources, pathways and environmental data Chief Scientist’s Group report August 2021 We are the Environment Agency. We protect and improve the environment. We help people and wildlife adapt to climate change and reduce its impacts, including flooding, drought, sea level rise and coastal erosion. We improve the quality of our water, land and air by tackling pollution. We work with businesses to help them comply with environmental regulations. A healthy and diverse environment enhances people's lives and contributes to economic growth. We can’t do this alone. We work as part of the Defra group (Department for Environment, Food & Rural Affairs), with the rest of government, local councils, businesses, civil society groups and local communities to create a better place for people and wildlife. Published by: Author: Emma Pemberton Environment Agency Horizon House, Deanery Road, Environment Agency’s Project Manager: Bristol BS1 5AH Mark Sinton www.gov.uk/environment-agency Citation: Environment Agency (2021) Poly- and © Environment Agency 2021 perfluoroalkyl substances (PFAS): sources, pathways and environmental All rights reserved. This document may data. Environment Agency, Bri be reproduced with prior permission of the Environment Agency. Further copies of this report are available from our publications catalogue: www.gov.uk/government/publications or our National Customer Contact Centre: 03708 506 506 Email: research@environment- agency.gov.uk 2 of 110 Research at the Environment Agency Scientific research and analysis underpins everything the Environment Agency does. It helps us to understand and manage the environment effectively. Our own experts work with leading scientific organisations, universities and other parts of the Defra group to bring the best knowledge to bear on the environmental problems that we face now and in the future. -

Maine Remedial Action Guidelines (Rags) for Contaminated Sites
Maine Department of Environmental Protection Remedial Action Guidelines for Contaminated Sites (RAGs) Effective Date: May 1, 2021 Approved by: ___________________________ Date: April 27, 2021 David Burns, Director Bureau of Remediation & Waste Management Executive Summary MAINE DEPARTMENT OF ENVIRONMENTAL PROTECTION 17 State House Station | Augusta, Maine 04333-0017 www.maine.gov/dep Maine Department of Environmental Protection Remedial Action Guidelines for Contaminated Sites Contents 1 Disclaimer ...................................................................................................................... 1 2 Introduction and Purpose ............................................................................................... 1 2.1 Purpose ......................................................................................................................................... 1 2.2 Consistency with Superfund Risk Assessment .............................................................................. 1 2.3 When to Use RAGs and When to Develop a Site-Specific Risk Assessment ................................. 1 3 Applicability ................................................................................................................... 2 3.1 Applicable Programs & DEP Approval Process ............................................................................. 2 3.1.1 Uncontrolled Hazardous Substance Sites ............................................................................. 2 3.1.2 Voluntary Response Action Program -

Octafluoropropane
Revised edition no : 4 SAFETY DATA SHEET ACCORDING TO REGULATION 1907/2006 Date : 08.10.2019 OCTAFLUOROPROPANE 1. IDENTIFICATION OF THE SUBSTANCE/COMPOUND AND COMPANY 1.1. Identification of the product Name: Octafluoropropane Chemical nomenclature: IUPAC name: Octafluoropropane Synonyms: Perfluoropropane, Khladon 218, R218 Molecular formula: С3F8 Structural formula Molar mass: 188,02 EC number: 200-941-9 (EINECS) REACH Pre-Registration Reference number 05-2114096818-30-0000 30/06/2008 C&L bulk notification Reference number 02-2119708805-36-0000 CAS number: 76-19-7 1.2. Use of substance/ Is used as reaction mass for plasmochemical etching of semiconducting compound materials, and as a refrigerant. Recommended use For industrial or professional use only, not for use in everyday life. 1.3. Company identification Manufacturer Joint Stock Company «HaloPolymer Perm» 614042, Russia, Perm, ul. Lasvinskaya 98 Phone № +7(342) 250-61-50 Web site: www.halopolymer.ru Only REACH representative in JSC «HaloPolymer Perm» (Submitting legal entity URALCHEM Assist EU: GmbH) Johannssenstrasse 10 30159, Hannover, Germany Tel: +49 511 45 99 444 1.4 Emergency telephone number: +7-342-282-85-45 (24 hours) Great Britain +44 (0) 203 394 9870 (24/7) USA 1-877 271 7077 2. HAZARDS IDENTIFICATION 2.1. Substance classification 2.1.1. Classification in accordance with the Regulation (EC) No 1272/2008 [CLP/GHS] Liquefied gas, H280 2.1.2. Classification in Not classified as a dangerous product. accordance with the Directive 67/548/EEC 2.2 Label elements Hazard pictograms 2.2.1 Labeling according to the Regulation (EC) No 1272/2008 [CLP/GHS] GHS04 Signal word: Warning Hazard statements: H280 (Contains gas under pressure; may explode if heated). -

United States Patent Office Patented Aug
y 2,847,481 United States Patent Office Patented Aug. 12, 1958 2 invention produces a mixture of these isomers from which the desired CsCl isomer may be separately re 2,847,481 covered in substantially pure form. Apparently, the PRODUCTION OF OCTACHELOROMETHYLENE preparation of octachloromethylenecyclopentene has CYCLOPENTENE been of only academic interest as no attempt has been made to obtain the material by an economic process Aylmer H. Maude and David S. Rosenberg, Niagara suitable for commercial manufacture. Falls, N.Y., assignors to Hooker Electrochemical Com Octachloromethylenecyclopentene is a valuable chem pany, Niagara Falls, N.Y., a corporation of New York ical intermediate, useful in the synthesis of various other No Drawing. Application August 9, 1954 0 chemicals having diverse uses in the commercial arts. Serial No. 448,736 For example, it may be used as the starting material for making perchlorofulvene by reacting it with aluminum 5 Claims. (CI. 260-648) shavings in the presence of freshly sublimed aluminum chloride in ether solution for a period of about 12 hours This invention is concerned with the production of 5 (see Roedig, Ann. 569, 161-183, (1950)). Also, various unsaturated cyclic chlorocarbons having the empirical ketones may be made from octachloromethylenecyclo formula CsCl and more particularly to the production pentene by reaction with sulfuric acid. of octachloromethylenecyclopentene. The process of It is the object of this invention to provide a method the present invention involves introducing a mixture for the production of octachloromethylenecyclopentene of a C chlorohydrocarbon containing at least three 20 by an economic process which has a direct and simple chlorine atoms and chlorine into a reaction Zone con procedure and which is readily adaptable to commercial taining a porous surface active catalyst maintained at operation. -

Dependent Modeling Approach Derived from Semi-Empirical Quantum Mechanical Calculations
3D-QSAR/QSPR Based Surface- Dependent Modeling Approach Derived From Semi-Empirical Quantum Mechanical Calculations 3D-QSAR/QSPR-basierter, oberflächenabhängiger Modellierungsansatz, abgeleitet von semi-empirischen quantenmechanischen Rechnungen Der Naturwissenschaftlichen Fakultät der Friedrich-Alexander-Universität Erlangen-Nürnberg Zur Erlangung des Doktorgrades Dr. rer. nat. vorgelegt von Marcel Youmbi Foka aus Kamerun Als Dissertation genehmigt von der Naturwissenschaftlichen Fakultät/ vom Fachbereich Chemie und Pharmazie der Friedrich-Alexander-Universität Erlangen-Nürnberg Tag der mündlichen Prüfung: 05.12.2018 Vorsitzender des Promotionsorgans: Prof. Dr. Georg Kreimer Gutachter/in: Prof. Dr. Tim Clark Prof. Dr. Birgit Strodel Dedication In memory of my late Mother Lucienne Metiegam, who the Lord has taken unto himself on May 3, 2009. My mother, light of my life, God rest her soul, had a special respect for my studies. She had always encouraged me to move forward. I sincerely regret the fact that today she cannot witness the culmination of this work. Maman, que la Terre de nos Ancêtres te soit légère! This is a special reward for Mr. Joseph Tchokoanssi Ngouanbe, who always supported me financially and morally. That he find here the expression of my deep gratitude. i ii Acknowledgements I would like to pay tribute to all those who have made any contribution, whether scientific or not, to help carry out this work. All my thanks go especially to Prof. Dr. Tim Clark, who gave me the opportunity and means to work in his research team. I am grateful to have had him not only supervise my work but also for his patience and for giving me the opportunity to explore this fascinating topic. -

UK PFAS Workshop
UK PFAS Workshop Day 1 – April 27th 2021 Agenda The diversity of PFAS What is the PFAS levels in the environment Morning concern? What do we know about the public health risks NGO and industry perspectives Assessing the potential PFAS legacy What do we know Afternoon Water industry chemical investigations about UK sources? EA evaluations of REACH-registered PFAS used in UK 2 The diversity of PFAS Presented by: Ian Cousins (Department of Environmental Science, Stockholm University) Text in footer 3 The Diversity of PFAS Ian T. Cousins Department of Environmental Science, Stockholm University, Sweden UK Environmental Agency, 27th April 2021 Definitions of PFAS • Buck et al. (2011) – first class definition – PFAS = “the highly fluorinated aliphatic substances that contain 1 or more C atoms on which all the H substituents … have been replaced by F atoms, in such a manner that they contain the perfluoroalkyl moiety CnF2n+1–” (has to contain at least -CF3) • Interstate Technology and Regulatory Council (ITRC) – Same definition as Buck et al. (2011), but n 2 (i.e. must contain at least CF3CF2−) • OECD: list of 4730 – “…contain a perfluoroalkyl moiety with three or more carbons (i.e. – CnF2n–, n ≥ 3) or a perfluoroalkylether moiety with two or more carbons (i.e. –CnF2nOCmF2m−, n and m ≥1).” • OECD: broader definition planned (unpublished) – “…the fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom…” i.e. substances are PFAS that have at least one -CF2- or -CF3 moiety in their structure 5 So how many -

Crystallinity Changes and Phase Transitions of Selected Pharmaceutical Solids with Processing
CRYSTALLINITY CHANGES AND PHASE TRANSITIONS OF SELECTED PHARMACEUTICAL SOLIDS WITH PROCESSING by MARION W.Y. WONG B.Sc. (Pharm), The University of British Columbia, 1988 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF’ PHILOSOPHY in THE FACULTY OF GRADUATE STUDIES Faàulty of Pharmaceutical Sciences Division of Pharmaceutics and Biopharmaceutics We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA November 1993 © Marion W.Y. Wong, 1993 _______________________ In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives, It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. (Signature) Department of The University of British Columbia Vancouver, Canada Date jt7’ / DE-6 (2/88) 11 ABSTRACT The solid state properties of drugs and pharmaceutical excipients can be significantly affected by processing (e.g. grinding, tabletting, heating and additive incorporation) and reflect structural changes within a solid. Such changes may involve alterations in both the chemical and physical nature of the crystal structure (e.g. hydrates), complete rearrangements of the same chemical components in three-dimensional space (e.g. polymorphs), or more subtle changes which involve neither the chemical composition nor the space lattice. These more subtle changes do not involve phase changes and are referred to as changes in the degree of crystallinity, X. -
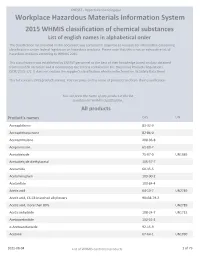
2015 WHMIS Classification : List of English Names in Alphabetical Order
CNESST - Répertoire toxicologique Workplace Hazardous Materials Information System 2015 WHMIS classification of chemical substances List of english names in alphabetical order The classification list provided in this document was compiled in response to requests for information concerning classifications under federal legislation on hazardous products. Please note that this is not an exhaustive list of hazardous products according to WHMIS 2015. This classification was established by CNESST personnel to the best of their knowledge based on data obtained from scientific literature and it incorporates the criteria contained in the Hazardous Products Regulations (SOR/2015-17). It does not replace the supplier's classification which can be found on its Safety Data Sheet. This list contains 2425 products names. You can press on the name of products to obtain their classification. You can press the name of any product in the list to obtain his WHMIS classification. All products Product's names CAS UN Acenaphthene 83-32-9 Acenaphthoquinone 82-86-0 Acenaphthylene 208-96-8 Acepromazine 61-00-7 Acetaldehyde 75-07-0 UN1089 Acetaldehyde diethylacetal 105-57-7 Acetamide 60-35-5 Acetaminophen 103-90-2 Acetanilide 103-84-4 Acetic acid 64-19-7 UN2789 Acetic acid, C6-C8 branched alkyl esters 90438-79-2 Acetic acid, more than 80% UN2789 Acetic anhydride 108-24-7 UN1715 Acetoacetanilide 102-01-2 o-Acetoacetaniside 92-15-9 Acetone 67-64-1 UN1090 2021-08-04 List of WHMIS controlled products 1 of 79 CNESST - Répertoire toxicologique Acetonitrile 75-05-8 UN1648