Performance in Delivering Clinical Research (Q3, 2016/17)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

European Iron Club 7
EUROPEAN IRON CLUB 7 - 10 MEETING IN April INNSBRUCK 2016 Programme Kein Eisen unter der Oberfläche Novartis Pharma GmbH Stella-Klein-Loew-Weg 17 | 1020 Wien www.novartispharma.at | +43 1 866 57-0 Erstellungsdatum 02/2016 | AT1602436490 CONTENTS Welcome 6 Committees 7 Masterclass in Iron Therapies 8 Thursday, 7 April 2016 European Iron Club Annual Meeting 10 Friday, 8 April 2016 European Iron Club Annual Meeting 20 Saturday, 9 April 2016 Scientific Programme 31 Kein Eisen Sunday, 10 April 2016 Innsbruck city map 34 unter der General Information 35 Exhibitors & Sponsors 40 Oberfläche Drug labels 41 Notes 42 Novartis Pharma GmbH 3 Stella-Klein-Loew-Weg 17 | 1020 Wien www.novartispharma.at | +43 1 866 57-0 Erstellungsdatum 02/2016 | AT1602436490 CONGRESS INFORMATION DATES CONGRESS ORGANISER Masterclass in Iron Therapies PCO TYROL CONGRESS Thursday, 7 April, 2016 MMag. Ina Kähler Mechthild Walter European Iron Club Annual Rennweg 3 Meeting 6020 Innsbruck Friday, 8 April – Saturday, 9 April, Austria 2016 T: +43 (0) 512 575600 F: +43 (0) 512 575607 Non HFE Hemochromatosis E: [email protected] Registry Meeting I: www.pco-tyrolcongress.at Sunday, 10 April, 2016 Meeting of Patient Organisations Sunday, 10 April, 2016 EXHIBITION MANAGEMENT AND SPONSORING VENUE (THU - SAT) S12! STUDIO12 GMBH CONGRESS INNSBRUCK Ralph Kerschbaumer Rennweg 3 Kaiser Josef Straße 9 6020 Innsbruck 6020 Innsbruck Austria Austria www.cmi.at T: +43 (0) 512 890438 F: +43 (0) 512 890438 15 E: [email protected] I: www.studio12.co.at VENUE (SUN) AUSTRIA TREND HOTEL Rennweg 12a -

How I Treat Myelofibrosis
From www.bloodjournal.org by guest on October 7, 2014. For personal use only. Prepublished online September 16, 2014; doi:10.1182/blood-2014-07-575373 How I treat myelofibrosis Francisco Cervantes Information about reproducing this article in parts or in its entirety may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://www.bloodjournal.org/site/subscriptions/index.xhtml Advance online articles have been peer reviewed and accepted for publication but have not yet appeared in the paper journal (edited, typeset versions may be posted when available prior to final publication). Advance online articles are citable and establish publication priority; they are indexed by PubMed from initial publication. Citations to Advance online articles must include digital object identifier (DOIs) and date of initial publication. Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved. From www.bloodjournal.org by guest on October 7, 2014. For personal use only. Blood First Edition Paper, prepublished online September 16, 2014; DOI 10.1182/blood-2014-07-575373 How I treat myelofibrosis By Francisco Cervantes, MD, PhD, Hematology Department, Hospital Clínic, IDIBAPS, University of Barcelona, Barcelona, Spain Correspondence: Francisco Cervantes, MD, Hematology Department, Hospital Clínic, Villarroel 170, 08036 Barcelona, Spain. Phone: +34 932275428. -

Tracks 1-9 David P Steensma, MD Select Excerpts from the Interview
INTERVIEW David P Steensma, MD Dr Steensma is Faculty Member in the Adult Leukemia Program at Dana-Farber Cancer Institute and Associate Professor of Medicine at Harvard Medical School in Boston, Massachusetts. Tracks 1-9 Track 1 Novel agents under investigation for Track 6 Case discussion: A 68-year-old man FLT3-ITD-mutated acute myeloid with postpolycythemia vera myelofi- leukemia (AML) brosis whose symptoms begin to recur Track 2 Activity and tolerability of the orally after 2 years of ruxolitinib therapy administered inhibitor of FLT3/AXL Track 7 Activity and toxicities of novel JAK gilteritinib (ASP2215) in AML inhibitors — pacritinib, momelotinib — Track 3 Recent developments in myelodys- in myeloproliferative disorders plastic syndromes (MDS) Track 8 Case discussion: A 65-year-old woman Track 4 Clinical experience with lenalidomide with hydroxyurea-resistant polycythemia for patients with MDS with and vera treated with ruxolitinib without del(5q) Track 9 Clinical experience with dosing and Track 5 Management of MDS in patients with continuation of ruxolitinib therapy disease progression on a hypomethyl- in patients experiencing treatment- ating agent associated cytopenias Select Excerpts from the Interview Tracks 1-2 DR LOVE: Would you discuss some of the most promising new agents and strate- gies under investigation for patients with acute myeloid leukemia (AML)? DR STEENSMA: One area of interest involves investigation of agents targeting FLT3 mutations, which are driver mutations commonly associated with AML. The 2 general classes of FLT3 mutations are internal tandem duplication (ITD) mutations and tyrosine kinase domain (TKD) mutations. Both constitutively activate the FLT3 receptor, but ITD mutations tend to be associated with more proliferative disease and a poorer prognosis, and they’re more common than TKD mutations. -

WO 2014/194127 Al 4 December 2014 (04.12.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/194127 Al 4 December 2014 (04.12.2014) P O P C T (51) International Patent Classification: Songyuan; c/o Plexxikon Inc., 9 1 Bolivar Drive, Berkeley, C07D 213/75 (2006.01) C07D 487/04 (2006.01) California 94710 (US). SPEVAK, Wayne; c/o Plexxikon C07D 417/04 (2006.01) A61K 31/519 (2006.01) Inc., 9 1 Bolivar Drive, Berkeley, California 94710 (US). C07D 471/04 (2006.01) HABETS, Gaston G.; c/o Plexxikon Inc., 9 1 Bolivar Drive, Berkeley, California 94710 (US). BURTON, Betsy; (21) International Application Number: c/o Plexxikon Inc., 9 1 Bolivar Drive, Berkeley, California PCT/US20 14/040076 94710 (US). (22) International Filing Date: (74) Agents: TANNER, Lorna L. et al; Sheppard Mullin 29 May 2014 (29.05.2014) Richter & Hampton LLP, 379 Lytton Avenue, Palo Alto, (25) Filing Language: English California 94301-1479 (US). (26) Publication Language: English (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, (30) Priority Data: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, 61/829,190 30 May 2013 (30.05.2013) US BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (71) Applicant: PLEXXIKON INC. [US/US]; 1 Bolivar DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, Drive, Berkeley, California 94710 (US). HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, (72) Inventors: ZHANG, Chao; c/o Plexxikon Inc., 9 1 Bolivar MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, Drive, Berkeley, California 94710 (US). -

Overview of Current Targeted Anti-Cancer Drugs for Therapy in Onco-Hematology
medicina Review Overview of Current Targeted Anti-Cancer Drugs for Therapy in Onco-Hematology Stefania Crisci 1 , Filomena Amitrano 2, Mariangela Saggese 1, Tommaso Muto 3, Sabrina Sarno 4, Sara Mele 1, Pasquale Vitale 1, Giuseppina Ronga 1, Massimiliano Berretta 5 and Raffaele Di Francia 6,* 1 Hematology-Oncology and Stem Cell Transplantation Unit, Istituto Nazionale Tumori, Fondazione “G. Pascale” IRCCS, 80131 Naples, Italy 2 Gruppo Oncologico Ricercatori Italiano GORI ONLUS, 33100 Pordenone, Italy 3 Hematology and Cellular Immunology (Clinical Biochemistry) A.O. dei Colli Monaldi Hospital, 80131 Naples, Italy 4 Anatomia Patologica, Istituto Nazionale Tumori, Fondazione “G. Pascale” IRCCS, 80131 Naples, Italy 5 Department of Medical Oncology, CRO National Cancer Institute, 33081 Aviano (PN), Italy 6 Italian Association of Pharmacogenomics and Molecular Diagnostics (IAPharmagen), 60125 Ancona, Italy * Correspondence: [email protected] Received: 12 May 2019; Accepted: 24 July 2019; Published: 28 July 2019 Abstract: The upgraded knowledge of tumor biology and microenviroment provides information on differences in neoplastic and normal cells. Thus, the need to target these differences led to the development of novel molecules (targeted therapy) active against the neoplastic cells’ inner workings. There are several types of targeted agents, including Small Molecules Inhibitors (SMIs), monoclonal antibodies (mAbs), interfering RNA (iRNA) molecules and microRNA. In the clinical practice, these new medicines generate a multilayered step in pharmacokinetics (PK), which encompasses a broad individual PK variability, and unpredictable outcomes according to the pharmacogenetics (PG) profile of the patient (e.g., cytochrome P450 enzyme), and to patient characteristics such as adherence to treatment and environmental factors. This review focuses on the use of targeted agents in-human phase I/II/III clinical trials in cancer-hematology. -

New Strategies in Myeloproliferative Neoplasms: the Evolving Genetic and Therapeutic Landscape Ami B
CCR New Strategies Clinical Cancer Research New Strategies in Myeloproliferative Neoplasms: The Evolving Genetic and Therapeutic Landscape Ami B. Patel1, Nadeem A. Vellore2, and Michael W. Deininger3 Abstract The classical BCR–ABL1-negative myeloproliferative neo- off-target toxicities and, as monotherapy, has shown limited plasms (MPN) include essential thrombocythemia (ET), poly- effects on mutant allele burden. In this review, we discuss the cythemia vera (PV), and myelofibrosis (MF). Although these genetic heterogeneity contributing to the pathogenesis of clonal disorders share certain clinical and genetic features, MF MPNs, focusing on novel driver and epigenetic mutations and in particular is distinct for its complex mutational landscape, how they relate to combination therapeutic strategies. We severe disease phenotype, and poor prognosis. The genetic discuss results from ongoing studies of new JAK inhibitors and complexity inherent to MF has made this disease extremely report on new drugs and drug combinations that have dem- challenging to treat. Pharmacologic JAK inhibition has proven onstrated success in early preclinical and clinical trials, includ- to be a transformative therapy in MPNs, alleviating symptom ing type II JAK inhibitors, antifibrotic agents, and telomerase burden and improving survival, but has been hampered by inhibitors. Clin Cancer Res; 22(5); 1037–47. Ó2016 AACR. Disclosure of Potential Conflicts of Interest M.W. Deininger reports receiving commercial research grants from Bristol-Myers Squibb, Celgene, Gilead, and Novartis, and is a consultant/ advisory board member for ARIAD Pharmaceuticals, Bristol-Myers Squibb, Incyte, Novartis, and Pfizer. No potential conflicts of interest were disclosed by the other authors. Editor's Disclosures The following editor(s) reported relevant financial relationships: J.L. -

Study Protocol: Amendment 3
(!') GILEAU CLINICAL STUDY PROTOCOL Study Title: A Phase 3, Randomized, Double-blind, Placebo-controlled Study of Gemcitabine and Nab-paclitaxel combined with Momelotinib in Subjects with Previously Unu·eated Metastatic Pancreatic Ductal Adenocarcinoma Preceded by a Dose-fmding, Lead-in Phase Sponsor: Gilead Sciences, Inc. 333 Lakeside Drive Foster City, CA 94404 USA IND Number: 120605 EudraCT Number: 2014-004480-20 ClinicalTrials.gov Identifier: NCT021 01021 Indication: Previously unu·eated metastatic pancreatic ductal adenocarcinoma Protocol ID: GS-US-370-1296 Clinical Trials Manager: Name: PPD Telephone: PPD Gilead Medical Monitor: Name: Peter Lee, MD, PhD Telephone: PPD Clinical Program Manager: Name: PPD Telephone: PPD Protocol Version/Date: Original: 11 Febmary 2014 Amendment 1: 14 March 2014 Amendment 2: 25 August 2014 Amendment 3: 16 July 2015 CONFIDENTIALITY STATEMENT The inf01mation contained in this document, pruiicularly unpublished data, is the prope1iy or under conu·ol of Gilead Sciences, Inc., and is provided to you in confidence as an investigator, potential investigator, or consultant, for review by you, your staff, and an applicable Institutional Review Board or Independent Ethics Committee. The infonnation is only to be used by you in connection with authorized clinical studies of the investigational dmg described in the protocol. You will not disclose any of the infonnation to others without written authorization from Gilead Sciences, Inc., except to the extent necessa1y to obtain infonned consent from those persons -

Karen Ortiz Cruz, M.D
Karen Ortiz Cruz, M.D. CURRICULUM VITAE Ironwood Cancer & Research Centers a division of Ironwood Physicians, PC PROFESSIONAL EXPERIENCE Ironwood Cancer & Research Centers Chandler, AZ Physician, Medical Oncology/Hematology September 2014 Waccamaw Oncology Private Practice Georgetown, SC Physician, Medical Oncology/Hematology December 2012 – June 2014 EDUCATION Post-Graduate Training: Medical University of South Carolina Charleston, SC Hematology/Oncology Fellow July 2011 – July 2014 Banner Good Samaritan Medical Center Phoenix, AZ Internal Medicine Intern/Resident June 2008 – June 2011 Education: Ross University School of Medicine Dominica, West Indies Doctor of Medicine September 2004 – June 2008 Foundation for Advanced Education in Sciences Bethesda, MD National Institutes of Health June 2002 – June 2004 University of Puerto Rico Cayey, PR Bachelor of Science August 1999 – June 2002 Major: Natural Science. Cum Laude CERTIFICATION/LICENSURE Board Certified, American Board of Internal Medicine August 2011 Board Eligible, ABIM Hematology and Medical Oncology, (certification upon completion of Fellowship in June 2014) Permanent License, South Carolina Board of Medical Examiners RESEARCH Post-Baccalaureate Research Student National Cancer Institute’s/Pediatric Oncology Branch, Bethesda, MD Preceptor Lee Helman, MD, PhD Research: Effects of CCI-779, an inhibitor of mTOR/S6Kl 4E-BPl pathway, on both primary tumor growth and metastasis tumor growth in rhabdomyosarcoma xenograft and mouse sarcoma model. 2003-2004 Post-Baccalaureate Research Student National Cancer Institute’s /Radiation Oncology Branch, Bethesda, MD Preceptor David Gius, M.D, PhD Research: Determine the function of two redox-sensitive transcriptional complex NF-κB and AP-1, which appear to be regulated by Thioredoxin Reductase and Thioredoxin. 2002-2003 Research Student Fellow University of Nebraska Medical Center- Eppley Cancer Institute, Omaha, NE Preceptor Robert Lewis, PhD Research: Construction of mice model with Kinase Suppressor of Ras (KSR) deletion. -

Protocol Alert
PROTOCOL ALERT New Clinical Trials Recently Added to the National Cancer Institute’s Database BLADDER CANCER Phase 1/1b Study to Evaluate the Safety Trial IDs: 556-15, NCI-2015-01502, and Tolerability of CPI-444 Alone and NCT02553447 Type: Biomarker/Laboratory analysis, A Study Of Avelumab In Patients in Combination With Atezolizumab in Treatment With Locally Advanced Or Metastatic Advanced Cancers Ibrutinib in Treating Minimal Residual Age: 18 to 75 Urothelial Cancer (JAVELIN Bladder Status: Active Disease in Patients With Chronic Trial IDs: UW14113, NCI-2015-02269, 100) Phase: Phase I Lymphocytic Leukemia After Front- 2015-0996, NCT02652468 Status: Active Type: Treatment Line Therapy Phase: Phase III Age: 18 and over Status: Active ESOPHAGEAL CANCER Type: Treatment Trial IDs: CPI-444-001, NCI-2016- Phase: Phase II Age: 18 and over 00227, NCT02655822 Type: Biomarker/Laboratory analysis, Proton Beam Radiation Therapy Trial IDs: B9991001, NCI-2016-00304, Treatment or Intensity-Modulated Radiation 2015-003262-86, JAVELIN Bladder 100, Circulating Tumor Cells in Operative Age: 18 and over Therapy in Treating Patients With NCT02603432 Blood in Patients With Bladder Cancer Trial IDs: MC1481, NCI-2015-02153, Esophageal Cancer Status: Not yet active NCT02649387 Status: Active A Study of Intravesical Apaziquone Phase: No phase specified Phase: Phase III as a Surgical Adjuvant in Patient Type: Biomarker/Laboratory analysis, Ibrutinib or Idelalisib in Treating Type: Treatment Undergoing TURBT Natural history/Epidemiology Patients With Persistent -

State-Of-The-Art Solutions for Myelofibrosis the Intersection of JAK Inhibitors, Allogeneic Transplant, and Other Strategies for Patient Care
State-of-the-Art Solutions for Myelofibrosis The Intersection of JAK Inhibitors, Allogeneic Transplant, and Other Strategies for Patient Care This session is open only to registrants of the 2020 Transplantation and Cellular Therapy (TCT) Meetings of ASTCT and CIBMTR in Orlando, FL. Disclosures Prithviraj Bose, MD, has a financial Jeanne M. Palmer, MD, has a financial interest/relationship or affiliation in the form of: interest/relationship or affiliation in the form of: Consultant and/or Advisor for Celgene Consultant and/or Advisor for CTI BioPharma Corporation; CTI BioPharma Corp.; Incyte Corp. Corporation; and Kartos Therapeutics, Inc. Grant/Research Support from Astellas Pharma US, Inc.; Blueprint Medicines Corporation; Celgene Corporation; CTI BioPharma Corp.; Incyte Corporation; Kartos Therapeutics, Inc.; NS Pharma,Inc.; Pfizer, Inc.; Promedior, Inc. and Constellation Pharmaceuticals. This CME activity is jointly provided by The Medical College of Wisconsin and PVI, PeerView Institute for Medical Education. This activity is supported by an educational grant from Celgene Corporation. Visit us at PeerView.com/MF2020 • Watch for the onDemand version in the coming weeks • Download the slides and Practice Aids • Apply for CME credit Join the conversation on Twitter @PeerView Need more information? Send an email to [email protected] Welcome, Introduction, and Baseline Assessment Jeanne M. Palmer, MD Associate Professor, Division of Hematology and Oncology Photo Pending Vice Chair and Section Lead, Division of Hematology Program Director, -

Quantitative Modeling and Analysis of Drug Screening Data for Personalized Cancer Medicine
UNIVERSITY OF HELSINKI FACULTY OF MEDICINE QUANTITATIVE MODELING AND ANALYSIS OF DRUG SCREENING DATA FOR PERSONALIZED CANCER MEDICINE Lenalidomide Momelotinib Imiquimod Tacedinaline Tofacitinib Temsirolimus Ruxolitinib Roscovitine Tofacitinib Entinostat Gefitinib RoscovitineRuxolitinib Refametinib Pimasertib Sirolimus Selumetinib Tandutinib EverolimusNilotinib PF−04691502 NVP−BEZ235 Trametinib Palbociclib Melphalan Belinostat CUDC−101 AZD8055 Erlotinib Gefitinib Momelotinib Tacedinaline Cediranib SNS−032 Levamisole PF−04691502 Motesanib Panobinostat NVP−AUY922 Palbociclib OSI−027 Erlotinib OSI−027 Axitinib BIIB021 Imatinib Alvespimycin Masitinib TemsirolimusVorinostat Ponatinib Sunitinib Dasatinib Tanespimycin Melphalan Sorafenib Masitinib Pazopanib Sirolimus NVP−SNSAUY922−032 Vatalanib Dasatinib Regorafenib Everolimus Sunitinib MGCD−265 Panobinostat Tivozanib AZD8055 Foretinib NVP−BEZ235 Regorafenib Entinostat Prednisolone Tivozanib Dexamethasone Vincristine Cediranib Belinostat Methylprednisolone Pazopanib CUDC100−101 100 Vinblastine 100 100 Refametinib −40−20 0 20 40 Vatalanib Vorinostat Pimasertib MK1775 Sorafenib MGCD−80265 80 Trametinib ABT−751 80 80 Vandetanib Logistic Selumetinib Axitinib Alvespimycin DSS Triethylenemelamine S−trityl−L−cysteine 60 function3 60 DSS / sDSS 60 AA 60 Canertinib Tanespimycin Mechlorethamine Camptothecin Crizotinib Ponatinib Mitomycin C Paclitaxel Fingolimod 40 IC50 40 Lapatinib Calculation 40 40 Afatinib Imiquimod Patupilone AddictionDrug response score 100 Slope Chlorambucil DSS Tandutinib BIIB021 -
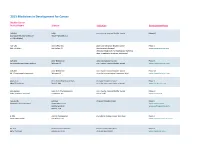
Adis R&D Insight
2015 Medicines in Development for Cancer Bladder Cancer Product Name Sponsor Indication Development Phase ABI-009 AADi non-muscle invasive bladder cancer Phase I/II (nanoparticle albumin-bound Pacific Palisades, CA mTOR inhibitor) ACP-196 Acerta Pharma platinum-refractory bladder cancer Phase II (Btk inhibitor) San Carlos, CA (combination therapy) www.acerta-pharma.com (see also head/neck, hematological, leukemia, lung, lymphoma, myeloma, pancreatic) ALT-801 Altor BioScience advanced bladder cancer, Phase II (immunotherapy fusion protein) Miramar, FL non-muscle invasive bladder cancer www.altorbioscience.com ALT-803 Altor BioScience non-muscle invasive bladder cancer Phase I/II (IL-15 superagonist complex) Miramar, FL (see also hematological, myeloma, skin) www.altorbioscience.com apatorsen OncoGenex Pharmaceuticals metastatic bladder cancer Phase II (Hsp27 inhibitor) Bothell, WA (see also lung, pancreatic, prostate) www.oncogenex.com apaziquone Spectrum Pharmaceuticals non-muscle invasive bladder cancer Phase III (DNA synthesis inhibitor) Henderson, NV (Fast Track) www.sppirx.com ASG-15ME Agensys relapsed bladder cancer Phase I (antibody drug conjugate) Santa Monica, CA www.agensys.com Seattle Genetics www.seattlegenetics.com Bothell, WA B-701 BioClin Therapeutics metastatic bladder cancer (2nd-line) Phase II (anti-FGFR3 mAb) San Ramon, CA www.bioclintherapeutics.com BC-819 BioCancell Therapeutics bladder cancer (2nd-line) Phase II (gene therapy) Jerusalem, Israel (see also pancreatic) www.biocancell.com Bladder Cancer Product Name Sponsor