Vitreous Anatomy, Aging, and Anomalous Posterior Vitreous Detachment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Meet the Choroid N Optometric Management Joe Pizzimenti, OD, FAAO O Scientific Advisory Boards [email protected] N Zeiss N Zeavision N Thrombogenics N Genentech
10/29/19 Financial Disclosures o Honoraria n Review of Optometry Meet The Choroid n Optometric Management Joe Pizzimenti, OD, FAAO o Scientific Advisory Boards [email protected] n Zeiss n Zeavision n Thrombogenics n Genentech Financial Disclosures Goals for This Course o Consulting Fees n Zeiss o Functional anatomy review n Zeavision n Choroid n Maculogix o Choroid examination and evaluation o Proprietary Interests o Case examples n None o Interactive o Stockholder: Zeavision Questions? 1 10/29/19 The Choroid The Choroid: Structure, o Located between the Function, and Evaluation sclera and the RPE n Extends from ora serrata to optic nerve o Pigmented/vascular tissue .75mm thick o Nourishes the RPE n Choroiocapillaris designed to leak o Absorbs light that passes through retina The Choriod RPE Bruch’s Membrane thickness o Loose connective tissue o Basal lamina of RPE o Melanocytes o Anterior collagenous o Choriocapillaris layer Mel. n Fenestrated endothelium o Elastic layer allows diffusion of o Posterior collagenous proteins CC layer n S__________ regulation o Basal lamina of CC BM n High blood flow endothelium n Very little O-2 extracted, o Contamination of so high venous O-2 Bruch’s can result in sclera d________, CNVM Nourishing the Retina Choroid Microstructure o 2 main sources of blood supply to retina: • Choriocapillaris o Choroidal BVs n Supplies outer retinal • Sattler’s layer layers, including PRs o CRA • Haller’s layer n 4 branches nourish inner retina • Supra - choroid n Run radially toward fovea 2 10/29/19 Imaging the Vascular Layers Imaging the Choroid of the Choroid WHAT IS ENHANCED Imaging the Choroid-EDI DEPTH OCT IMAGING? • EDI-OCT • Enhanced-depth imaging (EDI) OCT modifies the standard technique of image acquisition to better reveal the structural details of the choroid. -

Hyperopia Hyperopia
Hyperopia Hyperopia hyperopia hyperopia • Farsightedness, or hyperopia, • Farsightedness occurs if your eyeball is too as it is medically termed, is a short or the cornea has too little curvature, so vision condition in which distant objects are usually light entering your eye is not focused correctly. seen clearly, but close ones do • Its effect varies greatly, depending on the not come into proper focus. magnitude of hyperopia, the age of the individual, • Approximately 25% of the the status of the accommodative and general population is hyperopic (a person having hyperopia). convergence system, and the demands placed on the visual system. By Judith Lee and Gretchyn Bailey; reviewed by Dr. Vance Thompson; Flash illustration by Stephen Bagi 1. Cornea is too flap. hyperopia • In theory, hyperopia is the inability to focus and see the close objects clearly, but in practice many young hyperopics can compensate the weakness of their focusing ability by excessive use of the accommodation functions of their eyes. Hyperopia is a refractive error in • But older hyperopics are not as lucky as them. By which parallel rays of light aging, accommodation range diminishes and for 2. Axial is too short. entering the eye reach a focal older hyperopics seeing close objects becomes point behind the plane of the retina, while accommodation an impossible mission. is maintained in a state of relaxation. 1 Amplitude of Accommodation hyperopia Maximum Amplitude= 25-0.4(age) • An emmetropic eye for reading and other near Probable Amplitude= 18.5-.3(age) work, at distance of 16 in (40cm), the required amount of acc. -

Defining Emmetropia and Ametropia As a Function of Ocular Biometry II
SyntEyes: a Higher Order Statistical Eye Model for Healthy Eyes Jos J. Rozema,*† MSc PhD, Pablo Rodriguez,‡ MSc PhD, Rafael Navarro,‡ MSc PhD, Marie-José Tassignon*†, MD PhD *Dept. of Ophthalmology, Antwerp University Hospital, Edegem, Belgium † Dept. of Medicine and Health Sciences, Antwerp University, Wilrijk, Belgium ‡ICMA, Consejo Superior de Investigaciones Científicas-Universidad de Zaragoza, Facultad de Ciencias, Zaragoza, Spain Abstract Purpose: To present a stochastic eye model that simulates the higher order shape parameters of the eye, as well as their variability and mutual correlations. Methods: The biometry of 312 right eyes of 312 subjects were measured with an autorefractometer, a Scheimpflug camera, an optical biometer and a ray tracing aberrometer. The corneal shape parameters were exported as Zernike coefficients, which were converted into eigenvectors in order to reduce the dimensionality of the model. These remaining 18 parameters were modeled by fitting a sum of two multivariate Gaussians. Based on this fit an unlimited number of synthetic data sets (‘SyntEyes’) can be generated with the same distribution as the original data. After converting the eigenvectors back to the Zernike coefficients, the data may be introduced into ray tracing software. Results: The mean values of nearly all SyntEyes parameters was statistically equal to those of the original data (two one-side t test). The variability of the SyntEyes parameters was the same as for the original data for the most important shape parameters and intraocular distances, but showed significantly lower variability for the higher order shape parameters (F test) due to the eigenvector compression. The same was seen for the correlations between higher order shape parameters. -

PRESBYOND Laser Blended Vision Practical Guide
PRESBYOND Laser Blended Vision Practical Guide Disclaimer: This practical guide was produced independently by Dan Z Reinstein, MD MA(Cantab) FRCSC DABO FRCOphth FEBO1, 2, 3, 4 Glenn I Carp, MBBCh, FC Ophth (SA)1 Timothy J Archer, MA(Oxon), DipCompSci(Cantab)1, 4 Sharon Ritchie, BSc (Hons), MCOptom1 1 London Vision Clinic, London, UK 2 Department of Ophthalmology, Columbia University Medical Center, NY, USA 3 Centre Hospitalier National d’Ophtalmologie, Paris, France 4 Biomedical Science Research Institute, University of Ulster, Coleraine, Northern Ireland Financial Disclosure: Dr Reinstein is a consultant for Carl Zeiss Meditec (Carl Zeiss Meditec AG, Jena, Germany) and has a proprietary interest in the Artemis technology (ArcScan Inc, Golden, Colorado) through patents administered by the Center for Technology Licensing at Cornell University (CTL), Ithaca, New York. Dr Carp receives travel expenses from Carl Zeiss Meditec. The remaining authors have no proprietary or financial interest in the materials presented herein. Preoperative 1. Pre-operative testing protocol 2. Manifest refraction 3. Dominance testing 4. Laser Blended Vision tolerance assessment 5. What myopic target to expect 6. Laser Blended Vision explanation and patient counselling Postoperative 7. Postoperative evaluation 8. Postoperative visual course 9. Cross-blur management at final outcome 10. Appendix A – Preoperative tolerance test examples 11. Appendix B – Postoperative cross-blur and enhancement examples 2 1. Pre-operative testing protocol Highlighted topics are particularly relevant for PRESBYOND • History. Motivation for surgery, previous ocular • Cirrus OCT corneal and epithelial pachymetry. history (including detailed history of contact lens wear, • Undilated WASCA aberrometry. period of wear, type of lens, wear modality, last worn, • Ocular Response Analyser. -

Posterior Vitreous Detachment As Observed by Wide-Angle OCT Imaging
Posterior Vitreous Detachment as Observed by Wide-Angle OCT Imaging Mayuka Tsukahara, OD,1,* Keiko Mori, MD,1 Peter L. Gehlbach, MD, PhD,2 Keisuke Mori, MD, PhD1,3,4,* Purpose: Posterior vitreous detachment (PVD) plays an important role in vitreoretinal interface disorders. Historically, observations of PVD using OCT have been limited to the macular region. The purpose of this study is to image the wide-angle vitreoretinal interface after PVD in normal subjects using montaged OCT images. Design: An observational cross-sectional study. Participants: A total of 144 healthy eyes of 98 normal subjects aged 21 to 95 years (51.4Æ22.0 [mean Æ standard deviation]). Methods: Montaged images of horizontal and vertical OCT scans through the fovea were obtained in each subject. Main Outcome Measures: Montaged OCT images. Results: By using wide-angle OCT, we imaged the vitreoretinal interface from the macula to the periphery. PVD was classified into 5 stages: stage 0, no PVD (2 eyes, both aged 21 years); stage 1, peripheral PVD limited to paramacular to peripheral zones (88 eyes, mean age 38.9Æ16.2 years, mean Æ standard deviation); stage 2, perifoveal PVD extending to the periphery (12 eyes, mean age 67.9Æ8.4 years); stage 3, peripapillary PVD with persistent vitreopapillary adhesion alone (7 eyes, mean age 70.9Æ11.9 years); stage 4, complete PVD (35 eyes, mean age 75.1Æ10.1 years). All stage 1 PVDs (100%) were observed in the paramacular to peripheral region where the vitreous gel adheres directly to the cortical vitreous and retinal surface. After progression to stage 2 PVD, the area of PVD extends posteriorly to the perifovea and anteriorly to the periphery. -

Torpedo Maculopathy at the Site of the Fetal “Bulge”
SMALL CASE SERIES SECTION EDITOR: W. RICHARD GREEN, MD ward the foveola. This defect closely The flat, nonpigmented lesion mea- Torpedo Maculopathy resembles solitary CHRPE but dif- sured 2 mm horizontally and 1 mm at the Site of the fers in its nonrandom macular lo- vertically and was located 4 mm tem- Fetal “Bulge” cation and pointed torpedo shape.5-8 poral to the optic disc (Figure 2). In the few reported cases, there have Toxoplasmosis titer results were been no systemic associations. negative. Observation was advised. Torpedo maculopathy was discov- Herein, we describe 2 cases of tor- ered in 2 children as a pointed-oval pedo maculopathy and speculate as Comment. In 1992, Roseman and retinal pigment epithelial (RPE) de- to its embryogenesis. Gass3 described a 12-year-old boy fect in the temporal macula. This with a small, flat, circumscribed, oval congenital finding could be related Report of Cases. Case 1. On rou- RPE lesion in the temporal macula. to the fetal temporal macular “bulge” tine eye examination, a 3-year-old Additional reports confirmed the con- that normally occurs at 4 to 6 girl with fix-and-follow visual acu- sistent pointed oval configuration and months’ gestation at the same site. ity was discovered to have a tempo- macular location of this condition There are several congenital ral macular RPE defect with a (Table).5-8 Rigotti and associates7 re- anomalies of the RPE, including con- pointed-oval shape directed toward ported 3 cases of asymptomatic tor- genital hypertrophy of the RPE the foveola and hyperpigmented pedo maculopathy in a child and 2 (CHRPE), combined hamartoma of “frayed tail” appearance directed to- adults. -

Ciliary Body
Ciliary body S.Karmakar HOD Introduction • Ciliary body is the middle part of the uveal tract . It is a ring (slightly eccentric ) shaped structure which projects posteriorly from the scleral spur, with a meridional width varying from 5.5 to 6.5 mm. • It is brown in colour due to melanin pigment. Anteriorly it is confluent with the periphery of the iris (iris root) and anterior part of the ciliary body bounds a part of the anterior chamber angle. Introduction • Posteriorly ciliary body has a crenated or scalloped periphery, known as ora serrata, where it is continuous with the choroid and retina. The ora serrata exhibits forward extensions,known as dentate process, which are well defined on the nasal side and less so temporally. • Ciliary body has a width of approximately 5.9 mm on the nasal side and 6.7 mm on the temporal side. Extension of the ciliary body On the outside of the eyeball, the ciliary body extends from a point about 1.5 mm posterior to the corneal limbus to a point 6.5 to 7.5 mm posterior to this point on the temporal side and 6.5 mm posterior on the nasal side. Parts of ciliary body • Ciliary body, in cross section, is a triangular structure ( in diagram it can be compared as ∆ AOI). Outer side of the triangle (O) is attached with the sclera with suprachoroidal space in between. Anterior side of the triangle (A) forms part of the anterior & posterior chamber. In its middle, the iris is attached. The inner side of the triangle (I) is divided into two parts. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -
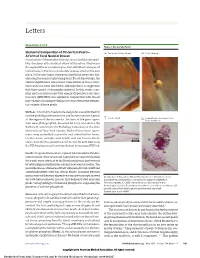
Elemental Composition of Ora Serrata Pearls—A Form of Focal Nodular
Letters RESEARCH LETTER Figure 1. Ora Serrata Pearls Elemental Composition of Ora Serrata Pearls— A Two pearls (50 and 10 µm) B Detailed image A Form of Focal Nodular Drusen Examination of the peripheral fundus may reveal discrete pearl- like structures at the furthest extent of the retina. They occur throughout the ora serrata region, but only where a tongue of retina tissue in the form of a dentate process overlies the pars plana. In the later stages, the pigment epithelial covering is lost, exposing the pearl as a glistening bead. To our knowledge, the clinical significance and precise composition of these struc- tures have not been described, although there is suggestion that they consist of drusenlike material. In this study, scan- ning electron microscopy with energy dispersive x-ray spec- troscopy (SEM-EDS) was applied in conjunction with classic histochemical staining techniques to characterize the elemen- tal content of these pearls. Methods | A formalin-fixed enucleated globe was submitted for routine pathological examination and found to contain 3 pearls C Calcified pearl D Pearl without attachment to the at the region of the ora serrata. Sections of the gross speci- Bruch membrane men were photographed, dissected (R.E.E.), and sent to the Barbara W. Streeten Ocular Pathology Laboratory at the State University of New York Upstate Medical University. Speci- mens were embedded in paraffin and submitted for hema- toxylin-eosin, periodic acid–Schiff, and van Gieson elastic 200 µm stains. A section was placed on a carbon stub for SEM-EDS using the FEI Aspex personal scanning electron microscope (FEI Inc). -

The Horizontal Raphe of the Human Retina and Its Watershed Zones
vision Review The Horizontal Raphe of the Human Retina and its Watershed Zones Christian Albrecht May * and Paul Rutkowski Department of Anatomy, Medical Faculty Carl Gustav Carus, TU Dresden, 74, 01307 Dresden, Germany; [email protected] * Correspondence: [email protected] Received: 24 September 2019; Accepted: 6 November 2019; Published: 8 November 2019 Abstract: The horizontal raphe (HR) as a demarcation line dividing the retina and choroid into separate vascular hemispheres is well established, but its development has never been discussed in the context of new findings of the last decades. Although factors for axon guidance are established (e.g., slit-robo pathway, ephrin-protein-receptor pathway) they do not explain HR formation. Early morphological organization, too, fails to establish a HR. The development of the HR is most likely induced by the long posterior ciliary arteries which form a horizontal line prior to retinal organization. The maintenance might then be supported by several biochemical factors. The circulation separate superior and inferior vascular hemispheres communicates across the HR only through their anastomosing capillary beds resulting in watershed zones on either side of the HR. Visual field changes along the HR could clearly be demonstrated in vascular occlusive diseases affecting the optic nerve head, the retina or the choroid. The watershed zone of the HR is ideally protective for central visual acuity in vascular occlusive diseases but can lead to distinct pathological features. Keywords: anatomy; choroid; development; human; retina; vasculature 1. Introduction The horizontal raphe (HR) was first described in the early 1800s as a horizontal demarcation line that extends from the macula to the temporal Ora dividing the temporal retinal nerve fiber layer into a superior and inferior half [1]. -

Distance Vision, Near Vision and Quality of Life Between Preferred Emmetropia and Residual Myopia in Monofocal Intraocular Lens Implantation - a Comparative Study
10-Comparative00212_3-PRIMARY.qxd 5/13/21 10:29 PM Page 340 ORIGINAL ARTICLE Distance vision, near vision and quality of life between preferred emmetropia and residual myopia in monofocal intraocular lens implantation - A Comparative study Jaafar Juanarita, MMed 1,2,3 , Abdul Rahman Siti-Khadijah, MBBS 1,2 , Bakiah Shaharuddin, PhD 4, Yaakub Azhany, MMed 1,2 1Department of Ophthalmology and Visual Sciences, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia, 2Ophthalmology Clinic, Hospital Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia, 3Hospital Sultanah Bahiyah, Alor Setar, Alor Setar, Kedah, 4Advanced Medical and Dental Institute, Universiti Sains Malaysia, Kepala Batas, Penang, Malaysia unoperated cataracts. 2 Currently, there is no effective ABSTRACT prevention for cataracts, and the only treatment is to remove Introduction: This study was done to evaluate the visual the cloudy lens. 3 acuity and quality of life in predicted emmetropia (EM) and predicted residual myopia (RM) patients following Intraocular lens (IOL) implantation is the commonest phacoemulsification with monofocal intraocular lens practice of visual rehabilitation after cataract surgery. implantation. Monofocal or fixed focal IOLs have only one focus at distance; thus, the placement of a monofocal IOL requires Materials and Methods: This prospective comparative study corrective lenses (spectacles) after surgery for near vision- was conducted in the ophthalmology clinic of the Universiti related tasks. Although no statistical difference was found Sains Malaysia Hospital, Kelantan, Malaysia. Overall, 139 between multifocal (MFIOL) and monofocal IOL with respect patients with senile cataract were randomised into EM and to achieving a post-operative best-corrected visual acuity RM groups. At three months post-operatively, patients were (BCVA) of 6/6, near vision was often found to be better with assessed for distance and near vision, as well as quality of MFIOL. -

The Nervous System: General and Special Senses
18 The Nervous System: General and Special Senses PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • Sensory information arrives at the CNS • Information is “picked up” by sensory receptors • Sensory receptors are the interface between the nervous system and the internal and external environment • General senses • Refers to temperature, pain, touch, pressure, vibration, and proprioception • Special senses • Refers to smell, taste, balance, hearing, and vision © 2012 Pearson Education, Inc. Receptors • Receptors and Receptive Fields • Free nerve endings are the simplest receptors • These respond to a variety of stimuli • Receptors of the retina (for example) are very specific and only respond to light • Receptive fields • Large receptive fields have receptors spread far apart, which makes it difficult to localize a stimulus • Small receptive fields have receptors close together, which makes it easy to localize a stimulus. © 2012 Pearson Education, Inc. Figure 18.1 Receptors and Receptive Fields Receptive Receptive field 1 field 2 Receptive fields © 2012 Pearson Education, Inc. Receptors • Interpretation of Sensory Information • Information is relayed from the receptor to a specific neuron in the CNS • The connection between a receptor and a neuron is called a labeled line • Each labeled line transmits its own specific sensation © 2012 Pearson Education, Inc. Interpretation of Sensory Information • Classification of Receptors • Tonic receptors