Eye Anatomy Slides File
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Experimental Glaucoma Model with Controllable Intraocular Pressure History Kayla R
www.nature.com/scientificreports OPEN Experimental glaucoma model with controllable intraocular pressure history Kayla R. Ficarrotta1, Youssef H. Mohamed1 & Christopher L. Passaglia1,2* Glaucoma-like neuropathies can be experimentally induced by disturbing aqueous outfow from the eye, resulting in intraocular pressure (IOP) changes that are variable in magnitude and time course and permanent in duration. This study introduces a novel method of glaucoma induction that ofers researchers round-the-clock measurement and reversible control of IOP for the frst time. One eye of Brown-Norway rats was implanted with a cannula tethered to a pressure sensor and aqueous reservoir. IOP was raised 10 mmHg for weeks-to-months in treated animals and unaltered in control animals. Counts of Brn3a-expressing retinal ganglion cells (RGCs) in implanted eyes were indistinguishable from non-implanted eyes in control animals and 15 ± 2%, 23 ± 4%, and 38 ± 4% lower in animals exposed to 2, 4, and 9 weeks of IOP elevation. RGC loss was greater in peripheral retina at 2 weeks and widespread at longer durations. Optic nerves also showed progressive degeneration with exposure duration, yet conventional outfow facility of implanted eyes was normal (24.1 ± 2.9 nl/min/mmHg) even after 9-weeks elevation. Hence, this infusion-based glaucoma model exhibits graded neural damage with unimpaired outfow pathways. The model further revealed a potentially-signifcant fnding that outfow properties of rat eyes do not remodel in response to chronic ocular hypertension. Glaucoma is a heterogeneous group of ocular disorders characterized by progressive and preferential loss of ret- inal ganglion cells (RGCs), resulting in visual feld defcits and ultimately blindness. -

MR Imaging of the Orbital Apex
J Korean Radiol Soc 2000;4 :26 9-0 6 1 6 MR Imaging of the Orbital Apex: An a to m y and Pat h o l o g y 1 Ho Kyu Lee, M.D., Chang Jin Kim, M.D.2, Hyosook Ahn, M.D.3, Ji Hoon Shin, M.D., Choong Gon Choi, M.D., Dae Chul Suh, M.D. The apex of the orbit is basically formed by the optic canal, the superior orbital fis- su r e , and their contents. Space-occupying lesions in this area can result in clinical d- eficits caused by compression of the optic nerve or extraocular muscles. Even vas c u l a r changes in the cavernous sinus can produce a direct mass effect and affect the orbit ap e x. When pathologic changes in this region is suspected, contrast-enhanced MR imaging with fat saturation is very useful. According to the anatomic regions from which the lesions arise, they can be classi- fied as belonging to one of five groups; lesions of the optic nerve-sheath complex, of the conal and intraconal spaces, of the extraconal space and bony orbit, of the cav- ernous sinus or diffuse. The characteristic MR findings of various orbital lesions will be described in this paper. Index words : Orbit, diseases Orbit, MR The apex of the orbit is a complex region which con- tains many nerves, vessels, soft tissues, and bony struc- Anatomy of the orbital apex tures such as the superior orbital fissure and the optic canal (1-3), and is likely to be involved in various dis- The orbital apex region consists of the optic nerve- eases (3). -

12 Retina Gabriele K
299 12 Retina Gabriele K. Lang and Gerhard K. Lang 12.1 Basic Knowledge The retina is the innermost of three successive layers of the globe. It comprises two parts: ❖ A photoreceptive part (pars optica retinae), comprising the first nine of the 10 layers listed below. ❖ A nonreceptive part (pars caeca retinae) forming the epithelium of the cil- iary body and iris. The pars optica retinae merges with the pars ceca retinae at the ora serrata. Embryology: The retina develops from a diverticulum of the forebrain (proen- cephalon). Optic vesicles develop which then invaginate to form a double- walled bowl, the optic cup. The outer wall becomes the pigment epithelium, and the inner wall later differentiates into the nine layers of the retina. The retina remains linked to the forebrain throughout life through a structure known as the retinohypothalamic tract. Thickness of the retina (Fig. 12.1) Layers of the retina: Moving inward along the path of incident light, the individual layers of the retina are as follows (Fig. 12.2): 1. Inner limiting membrane (glial cell fibers separating the retina from the vitreous body). 2. Layer of optic nerve fibers (axons of the third neuron). 3. Layer of ganglion cells (cell nuclei of the multipolar ganglion cells of the third neuron; “data acquisition system”). 4. Inner plexiform layer (synapses between the axons of the second neuron and dendrites of the third neuron). 5. Inner nuclear layer (cell nuclei of the bipolar nerve cells of the second neuron, horizontal cells, and amacrine cells). 6. Outer plexiform layer (synapses between the axons of the first neuron and dendrites of the second neuron). -

Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors
pharmaceutics Article Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors Hyeong Min Kim 1,†, Hyounkoo Han 2,†, Hye Kyoung Hong 1, Ji Hyun Park 1, Kyu Hyung Park 1, Hyuncheol Kim 2,* and Se Joon Woo 1,* 1 Department of Ophthalmology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam 13620, Korea; [email protected] (H.M.K.); [email protected] (H.K.H.); [email protected] (J.H.P.); [email protected] (K.H.P.) 2 Department of Chemical and Biomolecular Engineering, Sogang University, Seoul 04107, Korea; [email protected] * Correspondence: [email protected] (H.K.); [email protected] (S.J.W.); Tel.: +82-2-705-8922 (H.K.); +82-31-787-7377 (S.J.W.); Fax: +82-2-3273-0331 (H.K.); +82-31-787-4057 (S.J.W.) † These authors contributed equally to this work. Abstract: In this study, Retina-RPE-Choroid-Sclera (RCS) and RPE-Choroid-Sclera (CS) were prepared by scraping them off neural retina, and using the Ussing chamber we measured the average time– concentration values in the acceptor chamber across five isolated rabbit tissues for each drug molecule. We determined the outward direction permeability of the RCS and CS and calculated the neural retina permeability. The permeability coefficients of RCS and CS were as follows: ganciclovir, 13.78 ± 5.82 and 23.22 ± 9.74; brimonidine, 15.34 ± 7.64 and 31.56 ± 12.46; bevacizumab, 0.0136 ± 0.0059 and 0.0612 ± 0.0264 (×10−6 cm/s). -

Extraocular Muscles Orbital Muscles
EXTRAOCULAR MUSCLES ORBITAL MUSCLES INTRA- EXTRA- OCULAR OCULAR CILIARY MUSCLES INVOLUNTARY VOLUNTARY 1.Superior tarsal muscle. 1.Levator Palpebrae Superioris 2.Inferior tarsal muscle 2.Superior rectus 3.Inferior rectus 4.Medial rectus 5.Lateral rectus 6.Superior oblique 7.Inferior oblique LEVATOR PALPEBRAE SUPERIORIOS Origin- Inferior surface of lesser wing of sphenoid. Insertion- Upper lamina (Voluntary) - Anterior surface of superior tarsus & skin of upper eyelid. Middle lamina (Involuntary) - Superior margin of superior tarsus. (Superior Tarsus Muscle / Muller muscle) Lower lamina (Involuntary) - Superior conjunctival fornix Nerve Supply :- Voluntary part – Oculomotor Nerve Involuntary part – Sympathetic ACTION :- Elevation of upper eye lid C/S :- Drooping of upper eyelid. Congenital ptosis due to localized myogenic dysgenesis Complete ptosis - Injury to occulomotor nerve. Partial ptosis - disruption of postganglionic sympathetic fibres from superior cervical sympathetic ganglion. Extra ocular Muscles : Origin Levator palpebrae superioris Superior Oblique Superior Rectus Lateral Rectus Medial Rectus Inferior Oblique Inferior Rectus RECTUS MUSCLES : ORIGIN • Arises from a common tendinous ring knows as ANNULUS OF ZINN • Common ring of connective tissue • Anterior to optic foramen • Forms a muscle cone Clinical Significance Retrobulbar neuritis ○ Origin of SUPERIOR AND MEDIAL RECTUS are closely attached to the dural sheath of the optic nerve, which leads to pain during upward & inward movements of the globe. Thyroid orbitopathy ○ Medial & Inf.rectus thicken. especially near the orbital apex - compression of the optic nerve as it enters the optic canal adjacent to the body of the sphenoid bone. Ophthalmoplegia ○ Proptosis occur due to muscle laxity. Medial Rectus Superior Rectus Origin :- Superior limb of the tendonous ring, and optic nerve sheath. -

Meet the Choroid N Optometric Management Joe Pizzimenti, OD, FAAO O Scientific Advisory Boards [email protected] N Zeiss N Zeavision N Thrombogenics N Genentech
10/29/19 Financial Disclosures o Honoraria n Review of Optometry Meet The Choroid n Optometric Management Joe Pizzimenti, OD, FAAO o Scientific Advisory Boards [email protected] n Zeiss n Zeavision n Thrombogenics n Genentech Financial Disclosures Goals for This Course o Consulting Fees n Zeiss o Functional anatomy review n Zeavision n Choroid n Maculogix o Choroid examination and evaluation o Proprietary Interests o Case examples n None o Interactive o Stockholder: Zeavision Questions? 1 10/29/19 The Choroid The Choroid: Structure, o Located between the Function, and Evaluation sclera and the RPE n Extends from ora serrata to optic nerve o Pigmented/vascular tissue .75mm thick o Nourishes the RPE n Choroiocapillaris designed to leak o Absorbs light that passes through retina The Choriod RPE Bruch’s Membrane thickness o Loose connective tissue o Basal lamina of RPE o Melanocytes o Anterior collagenous o Choriocapillaris layer Mel. n Fenestrated endothelium o Elastic layer allows diffusion of o Posterior collagenous proteins CC layer n S__________ regulation o Basal lamina of CC BM n High blood flow endothelium n Very little O-2 extracted, o Contamination of so high venous O-2 Bruch’s can result in sclera d________, CNVM Nourishing the Retina Choroid Microstructure o 2 main sources of blood supply to retina: • Choriocapillaris o Choroidal BVs n Supplies outer retinal • Sattler’s layer layers, including PRs o CRA • Haller’s layer n 4 branches nourish inner retina • Supra - choroid n Run radially toward fovea 2 10/29/19 Imaging the Vascular Layers Imaging the Choroid of the Choroid WHAT IS ENHANCED Imaging the Choroid-EDI DEPTH OCT IMAGING? • EDI-OCT • Enhanced-depth imaging (EDI) OCT modifies the standard technique of image acquisition to better reveal the structural details of the choroid. -

The Distribution of Immune Cells in the Uveal Tract of the Normal Eye
THE DISTRIBUTION OF IMMUNE CELLS IN THE UVEAL TRACT OF THE NORMAL EYE PAUL G. McMENAMIN Perth, Western Australia SUMMARY function of these cells in the normal iris, ciliary body Inflammatory and immune-mediated diseases of the and choroid. The role of such cell types in ocular eye are not purely the consequence of infiltrating inflammation, which will be discussed by other inflammatory cells but may be initiated or propagated authors in this issue, is not the major focus of this by immune cells which are resident or trafficking review; however, a few issues will be briefly through the normal eye. The uveal tract in particular considered where appropriate. is the major site of many such cells, including resident tissue macro phages, dendritic cells and mast cells. This MACRO PHAGES review considers the distribution and location of these and other cells in the iris, ciliary body and choroid in Mononuclear phagocytes arise from bone marrow the normal eye. The uveal tract contains rich networks precursors and after a brief journey in the blood as of both resident macrophages and MHe class 11+ monocytes immigrate into tissues to become macro dendritic cells. The latter appear strategically located to phages. In their mature form they are widely act as sentinels for capturing and sampling blood-borne distributed throughout the body. Macrophages are and intraocular antigens. Large numbers of mast cells professional phagocytes and play a pivotal role as are present in the choroid of most species but are effector cells in cell-mediated immunity and inflam virtually absent from the anterior uvea in many mation.1 In addition, due to their active secretion of a laboratory animals; however, the human iris does range of important biologically active molecules such contain mast cells. -
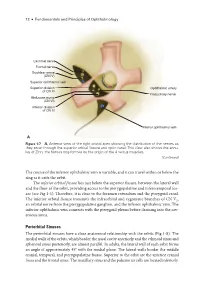
Periorbital Sinuses the Periorbital Sinuses Have a Close Anatomical Relationship with the Orbits (Fig 1-8)
12 ● Fundamentals and Principles of Ophthalmology Lacrimal nerve Frontal nerve Trochlear nerve (CN IV) Superior ophthalmic vein Superior division Ophthalmic artery of CN III Nasociliary nerve Abducens nerve (CN VI) Inferior division of CN III Inferior ophthalmic vein A Figure 1-7 A, Anterior view of the right orbital apex showing the distribution of the nerves as they enter through the superior orbital fissure and optic canal. This view also shows the annu- lus of Zinn, the fibrous ring formed by the origin of the 4 rectus muscles. (Continued) The course of the inferior ophthalmic vein is variable, and it can travel within or below the ring as it exits the orbit. The inferior orbital fissure lies just below the superior fissure, between the lateral wall and the floor of the orbit, providing access to the pterygopalatine and inferotemporal fos- sae (see Fig 1-1). Therefore, it is close to the foramen rotundum and the pterygoid canal. The inferior orbital fissure transmits the infraorbital and zygomatic branches of CN V2, an orbital nerve from the pterygopalatine ganglion, and the inferior ophthalmic vein. The inferior ophthalmic vein connects with the pterygoid plexus before draining into the cav- ernous sinus. Periorbital Sinuses The periorbital sinuses have a close anatomical relationship with the orbits (Fig 1-8). The medial walls of the orbits, which border the nasal cavity anteriorly and the ethmoid sinus and sphenoid sinus posteriorly, are almost parallel. In adults, the lateral wall of each orbit forms an angle of approximately 45° with the medial plane. The lateral walls border the middle cranial, temporal, and pterygopalatine fossae. -

Microscopic Anatomy of the Eye Dog Cat Horse Rabbit Monkey Richard R Dubielzig Mammalian Globes Mammalian Phylogeny General Anatomy Dog
Microscopic Anatomy of the eye Dog Cat Horse Rabbit Monkey Richard R Dubielzig Mammalian globes Mammalian Phylogeny General Anatomy Dog Arterial Blood Vessels of the Orbit General Anatomy Dog * Horizontal section Long Posterior Ciliary a. Blood enters the globe Short Post. Ciliary a Long Post. Ciliary a. Anterior Ciliary a. Blood Supply General Anatomy Dog Major arterial circle of the iris Orbital Anatomy Dog Brain Levator Dorsal rectus Ventral rectus Zygomatic Lymph node Orbital Anatomy Dog Orbital Anatomy Dog Cartilaginous trochlea and the tendon of the dorsal oblique m. Orbital Anatomy Dog Rabbit Orbital Anatomy Dog Zygomatic salivary gland mucinous gland Orbital Anatomy Dog Gland of the Third Eyelid Eye lids (dog) Eye lids (dog) Meibomian glands at the lid margin Holocrine secretion Eye lids (primate) Upper tarsal plate Lower tarsal plate Eye lids (rabbit) The Globe The Globe Dog Cat Orangutan Diurnal Horse Diurnal Cornea Epithelium Stromal lamellae Bowman’s layer Dolphin Descemet’s m Endothelium TEM of surface epithelium Cornea Doubling of Descemet’s Vimentin + endothelium Iris Walls: The vertebrate eye Iris Sphincter m. Dilator m Blue-eye, GFAP stain Iris Collagen Iris Cat Sphinctor m. Dilator m. Iris Cat Phyomelanocytes Iris Equine Corpora nigra (Granula iridica) seen in ungulates living without shade Ciliary body Pars plicata Ciliary muscle Pars plana Ciliary body Zonular ligaments Ciliary body Primarily made of fibrillin A major component of elastin Ciliary body Alcian Blue staining acid mucopolysaccharides: Hyaluronic acid Ciliary -

Japanese Journal of Ophthalmology Vol.43 No.4
Autonomic Nerves Containing Substance P in the Aqueous Outflow Channels and Scleral Spur of the Guinea Pig Mariko Sasamoto, Hai-Bo Chen and Shigeo Tsukahara Department of Ophthalmology, Yamanashi Medical University, Tamaho, Yamanashi, Japan Purpose: To study the innervation of the aqueous outflow channels and scleral spur by auto- nomic nerves containing substance P. Methods: The experiments were conducted on guinea pigs. Immunohistochemical tech- niques and capsaicin-ablation of the sensory nerves were used to investigate nerves contain- ing substance P at the light and electron microscopic level. Results: Nerves containing substance P were observed in the aqueous outflow channels and scleral spur regions. The fine structures of these nerves had a similar pattern in those regions, and the labeled elements had abundant small vesicles, a few large vesicles, and numerous neurotubuli. Following capsaicin treatment, these nerves remained intact and no degener- ated substance P-like immunoreactive nerves were found. Conclusions: Nerves containing substance P are most likely of autonomic origin in view of their ultrastructural features. These nerves innervate the aqueous outflow channels and scleral spur, and are probably important for neurogenic influences on the intraocular pres- sure by the autonomic nervous system. Jpn J Ophthalmol 1999;43:272–278 © 1999 Japa- nese Ophthalmological Society Key Words: Aqueous outflow channels, capsaicin, guinea pig, immunohistochemistry, substance P. Introduction The scleral spur region should also be considered 4,5 Aqueous outflow channels and the scleral spur because it plays a part in outflow regulation. The human scleral spur contains elastic tissue, similar to play important roles in the regulation of intraocular sclera, so that changes in the IOP might affect these pressure (IOP), and are known to have varied pepti- 5 dergic innervation, which have received special at- tissues. -

Passport to Success
The following terms and other boldface terms in the chapter are defined in the Glossary accommodation choroid After careful study of this chapter, you should be able to: cochlea conjunctiva 1. Describe the function of the sensory system convergence 2. Differentiate between the special and general senses and give examples of each cornea 3. Describe the structure of the eye gustation 4. List and describe the structures that protect the eye lacrimal apparatus 5. Define refraction and list the refractive parts of the eye lens (crystalline lens) 6. Differentiate between the rods and the cones of the eye olfaction 7. Compare the functions of the extrinsic and intrinsic muscles of organ of Corti the eye ossicle 8. Describe the nerve supply to the eye proprioceptor 9. Describe the three divisions of the ear refraction 10. Describe the receptor for hearing and explain how it functions retina 11. Compare static and dynamic equilibrium and describe the sclera location and function of these receptors semicircular canal 12. Explain the function of proprioceptors sensory adaptation 13. List several methods for treatment of pain sensory receptor 14. Describe sensory adaptation and explain its value tympanic membrane 15. Show how word parts are used to build words related to the vestibule sensory system (see Word Anatomy at the end of the chapter) vitreous body PASSport to Success Visit thePoint or see the Student Resource CD in the back of this book for definitions and pronun- ciations of key terms as well as a pretest for this chapter. ® Paul’s Second Case: Seeing More of the Sun’s Effects aul glanced once again at the postcard condition, and it does have a hereditary fac- sitting on his entranceway table as he ar- tor.” The doctor dilated Paul’s eyes with drops Prived home in the evening. -

Foveola Nonpeeling Internal Limiting Membrane Surgery to Prevent Inner Retinal Damages in Early Stage 2 Idiopathic Macula Hole
Graefes Arch Clin Exp Ophthalmol DOI 10.1007/s00417-014-2613-7 RETINAL DISORDERS Foveola nonpeeling internal limiting membrane surgery to prevent inner retinal damages in early stage 2 idiopathic macula hole Tzyy-Chang Ho & Chung-May Yang & Jen-Shang Huang & Chang-Hao Yang & Muh-Shy Chen Received: 29 October 2013 /Revised: 26 February 2014 /Accepted: 5 March 2014 # Springer-Verlag Berlin Heidelberg 2014 Abstract Keywords Fovea . Foveola . Internal limiting membrane . Purpose The purpose of this study was to investigate and macular hole . Müller cell . Vitrectomy present the results of a new vitrectomy technique to preserve the foveolar internal limiting membrane (ILM) during ILM peeling in early stage 2 macular holes (MH). Introduction Methods The medical records of 28 consecutive patients (28 eyes) with early stage 2 MH were retrospectively reviewed It is generally agreed that internal limiting membrane (ILM) and randomly divided into two groups by the extent of ILM peeling is important in achieving closure of macular holes peeing. Group 1: foveolar ILM nonpeeling group (14 eyes), (MH) [1]. An autopsy study of a patient who had undergone and group 2: total peeling of foveal ILM group (14 eyes). A successful MH closure showed an area of absent ILM sur- donut-shaped ILM was peeled off, leaving a 400-μm-diameter rounding the sealed MH [2]. ILM over foveola in group 1. The present ILM peeling surgery of idiopathic MH in- Results Smooth and symmetric umbo foveolar contour was cludes total removal of foveolar ILM. However, removal of restored without inner retinal dimpling in all eyes in group 1, all the ILM over the foveola causes anatomical changes of the but not in group 2.