Nematodes of the Eurasian Wigeon (Anas Penelope) and the Common Teal (A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Information Sheet on Ramsar Wetlands (RIS)
Information Sheet on Ramsar Wetlands (RIS) Categories approved by Recommendation 4.7 (1990), as amended by Resolution VIII.13 of the 8th Conference of the Contracting Parties (2002) and Resolutions IX.1 Annex B, IX.6, IX.21 and IX. 22 of the 9th Conference of the Contracting Parties (2005). Notes for compilers: 1. The RIS should be completed in accordance with the attached Explanatory Notes and Guidelines for completing the Information Sheet on Ramsar Wetlands. Compilers are strongly advised to read this guidance before filling in the RIS. 2. Further information and guidance in support of Ramsar site designations are provided in the Strategic Framework for the future development of the List of Wetlands of International Importance (Ramsar Wise Use Handbook 7, 2nd edition, as amended by COP9 Resolution IX.1 Annex B). A 3rd edition of the Handbook, incorporating these amendments, is in preparation and will be available in 2006. 3. Once completed, the RIS (and accompanying map(s)) should be submitted to the Ramsar Secretariat. Compilers should provide an electronic (MS Word) copy of the RIS and, where possible, digital copies of all maps. 1. Name and address of the compiler of this form: FOR OFFICE USE ONLY. DD MM YY Joint Nature Conservation Committee Monkstone House City Road Designation date Site Reference Number Peterborough Cambridgeshire PE1 1JY UK Telephone/Fax: +44 (0)1733 – 562 626 / +44 (0)1733 – 555 948 Email: [email protected] 2. Date this sheet was completed/updated: Designated: 28 November 1985 3. Country: UK (England) 4. Name of the Ramsar site: Martin Mere 5. -

Rapid Risk Assessment on Incursion of HPAI H5N8 Into Housed Or Not Housed Poultry Flocks and Captive Birds
Rapid risk assessment on incursion of HPAI H5N8 into housed or not housed poultry flocks and captive birds 29 January 2021 Situation as at 26 January 2021 © Crown copyright 2021 You may re-use this information (excluding logos) free of charge in any format or medium, under the terms of the Open Government Licence v.3. To view this licence visit www.nationalarchives.gov.uk/doc/open-government-licence/version/3/ or email [email protected] This publication is available at www.gov.uk/government/publications Any enquiries regarding this publication should be sent to: [email protected] www.gov.uk/defra 2 Contents Summary ............................................................................................................................................. 4 Introduction ........................................................................................................................................ 6 Hazard Identification ......................................................................................................................... 10 Previous outbreaks of HPAI H5N8: ................................................................................................... 12 Current Situation ............................................................................................................................... 12 Risk Question .................................................................................................................................... 16 Risk Levels .................................................................................................................................... -

Red-Breasted Goose
Urgent preliminary assessment of ornithological data relevant to spread of Avian Influenza in Europe Ward Hagemeijer Wetlands International Commissioned by DG Environment to Wetlands International and Euring Data needs for risk assessment: ornithology (virology) Quantified risk assessment: Research and Monitoring programme A. Birds as a vector 1.Quantified bird migration information: satellite telemetry, ringing data analysis, count data analysis 2.Quantified frequency of occurrence of virus in wild birds: surveillance 3.Ecology of virus in wild birds: virological research on wild birds B. Impact on wild bird populations Different components of the project Activities to be undertaken: • identification of Higher Risk Species (HRS) • analysis of their migration routes (ringing and count data) • identification of concentration and mixing sites • rapid assessment planning for wetland sites Analysis of higher risk species Identification of HRS on basis of: • occurence of LPAI viruses • ecology and behaviour • contact risk with poultry • numbers within EU In collaboration with David Stroud and Rowena Langston Occurrence of LPAI in wild birds species Results 1999 – 2004 Erasmus University Netherlands Species N Tested N PCR+ (%) N Egg + Mallard 6822 489 (7.2) 267 Eurasian Wigeon 1470 22 (1.5) 4 Common Teal 670 18 (2.7) 4 Northern Pintail 135 4 (3.0) 1 Northern Shoveler 90 1 (1.1) 1 Shelduck, Eider, Gadwall, Tufted, Garganey 238 0 (0.0) 0 Greater White-fronted Goose 1696 19 (1.1) 5 Greylag Goose 303 8 (2.6) 4 Brent, Barnacle, Bean, Egyptian, Canada, Pink-f 1202 0 (0.0) 0 Black-headed Gull 993 10 (1.0) 6 Common, Herring, Black-backed, Kittiwake 1976 0 (0.0) 0 Guillemot 698 3 (0.4) 1 Other birds 10909 0 0 + + + 27204 574 (2.1) 295 Selection of taxonomic groups • Anseriformes and Charadriiformes, • Migratory • Occurring in Europe. -
Greater White-Fronted Goose
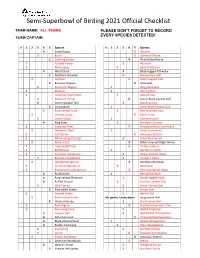
Semi-Superbowl of Birding 2021 Official Checklist TEAM NAME: ALL TEAMS PLEASE DON’T FORGET TO RECORD EVERY SPECIES DETECTED! TEAM CAPTAIN: √ 1 2 3 4 5 Species √ 1 2 3 4 5 Species 4 Snow Goose 5 Dovekie 3 Brant 5 Common Murre 5 Cackling Goose 4 Thick-billed Murre 1 Canada Goose 3 Razorbill 1 Mute Swan 2 Black Guillemot 4 Wood Duck 3 Black-legged Kittiwake 5 Northern Shoveler 3 Bonaparte’s Gull 2 Gadwall 4 Black-headed Gull 5 Eurasian Wigeon 5 Little Gull 3 American Wigeon 1 Ring-billed Gull 1 Mallard 1 Herring Gull 1 American Black Duck 2 Iceland Gull 3 Northern Pintail 4 Lesser Black-backed Gull 3 Green-winged Teal 3 Glaucous Gull 5 Canvasback 1 Great Black-backed Gull 4 Ring-necked Duck 2 Red-throated Loon 2 Greater Scaup 5 Pacific Loon 3 Lesser Scaup 1 Common Loon 4 King Eider 2 Northern Gannet 1 Common Eider 4 Double-crested Cormorant 2 Harlequin Duck 1 Great Cormorant 1 Surf Scoter 5 American Bittern 1 White-winged Scoter 3 Great Blue Heron 2 Black Scoter 4 Black-crowned Night-Heron 1 Long-tailed Duck 4 Turkey Vulture 1 Bufflehead 1 Northern Harrier 1 Common Goldeneye 3 Sharp-shinned Hawk 3 Barrow's Goldeneye 3 Cooper’s Hawk 2 Hooded Merganser 4 Northern Goshawk 1 Common Merganser 2 Bald Eagle 1 Red-breasted Merganser 4 Red-shouldered Hawk 4 Ruddy Duck 1 Red-tailed Hawk 4 Ring-necked Pheasant 3 Rough-legged hawk 4 Ruffed Grouse 2 Eastern Screech-Owl 3 Wild Turkey 3 Great Horned Owl 5 Pied-billed Grebe 3 Snowy Owl 1 Horned Grebe 3 Barred Owl 2 Red-necked Grebe No points. -

Handbook of Waterfowl Behavior: Tribe Anatini (Surface-Feeding Ducks)
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Handbook of Waterfowl Behavior, by Paul Johnsgard Papers in the Biological Sciences January 1965 Handbook of Waterfowl Behavior: Tribe Anatini (Surface-feeding Ducks) Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/bioscihandwaterfowl Part of the Ornithology Commons Johnsgard, Paul A., "Handbook of Waterfowl Behavior: Tribe Anatini (Surface-feeding Ducks)" (1965). Handbook of Waterfowl Behavior, by Paul Johnsgard. 16. https://digitalcommons.unl.edu/bioscihandwaterfowl/16 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Handbook of Waterfowl Behavior, by Paul Johnsgard by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Subfamily Anatinae 125 Aix. During extreme excitement the male will often roll his head on his back, or even bathe. I have not observed Preening-behind-the- wing, but W. von de Wall (pers. comm.) has observed a male per- form it toward a female. Finally, Wing-flapping appears to be used as a display by males, and it is especially conspicuous because each sequence of it is ended by a rapid stretching of both wings over the back in a posture that makes visible the white axillary feathers, which contrast sharply with the black underwing surface. Copulatory behavior. Precopulatory behavior consists of the male swimming up to the female, his neck stretched and his crest de- pressed, and making occasional Bill-dipping movements. He then suddenly begins to perform more vigorous Head-dipping movements, and the female, if receptive, performs similar Bill-dipping or Head- dipping movements. -

Cotswold Water Park Species Check List
Your local charity working for Record your Wildlife Sightings! wildlife and people Collecting biological records can provide us with important information about species and habitat distribution and abundance within the Cotswold Water Park. This information is made available to local planning authorities and conservation organisations and can help them to make decisions about future management. By submitting your sightings you can help to safeguard your local wildlife! So if you are spending time out and about in the Cotswold Water Park we would like to hear CotswoldCotswold WaterWater ParkPark about what you have seen! You can use the form below to record your data. Please include the species (common name is fine) and the location (such as lake number, reserve name or 6 figure SpeciesSpecies CheckCheck ListList grid reference if known). The completed form can then be posted to us at: Cotswold Water Park Trust, Manor Farm, Down Ampney, Cirencester, GL7 5QF We look forward to hearing from you soon! Species Location Date Comments Name:............................................................................................................................................ E-mail:........................................................................................................................................... Telephone:...................................................................................................................................... www.waterpark.org 01793 752730 Rachel Budworth Version Number: 03 December 2012 -

HUNTING-SEASONS.Pdf
HUNTING SEASON AUGUST 1 - JULY 31 AUG SEP OCT NOV DEC JAN FEB MAR APR MAY JUN JUL White tailed deer (Odocoileus virginianus) Open season Sep 1 - Feb 15 Roedeer (Capreolus capreolus) Open season Sep 1 - Feb 15 Roe buck May 5 - Jun 15 Moose (alces alces) Open season Sep 1 - Jan 15 Fallow deer (Dama dama) Open season Sep 1 - Jan 31 Mouflon (Ovis orientalis musimon) Open season Sep 1 - Jan 31 Wild boar (Sus scrofa) Open season all year round. Females with piglets are protected Mar 1 - Jul 31 Caribou (Rangifer tarandus) Open season Sep 30 - Jan 31 Brown hare (Lepus europaeus) Open season Sep 1 - Feb 28 Mountain hare (Lepus timidus) Open season Sep 1 - Feb 28 Rabbit (Oryctolagus cuniculus) Open season Sep 1 - Feb 28 European beaver (Castor fiber) Open season Aug 20 - Apr 30 North American beaver (Castor canadensis) Open season Aug 20 - Apr 30 Muskrat (Ondatra zibethicus) Open season Oct 1 - May 19 Red squirrel (Sciurus vulgaris) Nov 1-Feb 31 Brown bear (Ursus arctos) Open season Aug 20 - Oct 31 Grey wolf (Canis lupus) Lynx (Lynx lynx) Dec 1 - Feb 28 Wolverine (Gulo gulo) Grey seal (Halichoerus grypus) Open season Apr 16 - Dec 31 Open season Apr 16 - Dec 31 Ringed seal (Pusa hispida botnica) Open season Apr 16 - Dec 31 Open season Apr 16 - Dec 31 Red fox (Vulpes vulpes) Open season Aug 1 - Apr 14 Raccoon dog (Nyctereutes procyonoides) Open season all year round. Females with cubs are protected between May 1 - July 31 Badger (Meles meles) Open season Aug 1 - Mar 31 American Mink (Neovison vison) Open season all year round. -

(2007): Birds of the Aleutian Islands, Alaska Please
Bold* = Breeding Sp Su Fa Wi Bold* = Breeding Sp Su Fa Wi OSPREYS FINCHES Osprey Ca Ca Ac Brambling I Ca Ca EAGLES and HAWKS Hawfinch I Ca Northern Harrier I I I Common Rosefinch Ca Eurasian Sparrowhawk Ac (Ac) Pine Grosbeak Ca Bald Eagle* C C C C Asian Rosy-Finch Ac Rough-legged Hawk Ac Ca Ca Gray-crowned Rosy-Finch* C C C C OWLS (griseonucha) Snowy Owl I Ca I I Gray-crowned Rosy-Finch (littoralis) Ac Short-eared Owl* R R R U Oriental Greenfinch Ca FALCONS Common Redpoll I Ca I I Eurasian Kestrel Ac Ac Hoary Redpoll Ca Ac Ca Ca Merlin Ca I Red Crossbill Ac Gyrfalcon* R R R R White-winged Crossbill Ac Peregrine Falcon* (pealei) U U C U Pine Siskin I Ac I SHRIKES LONGSPURS and SNOW BUNTINGS Northern Shrike Ca Ca Ca Lapland Longspur* Ac-C C C-Ac Ac CROWS and JAYS Snow Bunting* C C C C Common Raven* C C C C McKay's Bunting Ca Ac LARKS EMBERIZIDS Sky Lark Ca Ac Rustic Bunting Ca Ca SWALLOWS American Tree Sparrow Ac Tree Swallow Ca Ca Ac Savannah Sparrow Ca Ca Ca Bank Swallow Ac Ca Ca Song Sparrow* C C C C Cliff Swallow Ca Golden-crowned Sparrow Ac Ac Barn Swallow Ca Dark-eyed Junco Ac WRENS BLACKBIRDS Pacific Wren* C C C U Rusty Blackbird Ac LEAF WARBLERS WOOD-WARBLERS Bold* = Breeding Sp Su Fa Wi Wood Warbler Ac Yellow Warbler Ac Dusky Warbler Ac Blackpoll Warbler Ac DUCKS, GEESE and SWANS Kamchatka Leaf Warbler Ac Yellow-rumped Warbler Ac Emperor Goose C-I Ca I-C C OLD WORLD FLYCATCHERS "HYPOTHETICAL" species needing more documentation Snow Goose Ac Ac Gray-streaked Flycatcher Ca American Golden-plover (Ac) Greater White-fronted Goose I -

Eligible Species for the Junior Duck Stamp Competition
Eligible Species for the Junior Duck Stamp Competition Your entry should feature a live portrayal featuring at least one of the species below. Mute swans, loons, grebes, coots and other such waterbirds are not permitted species. For the contest, you may do ones not found in Maine Species found in Maine ● Snow Goose, including blue phase (Anser caerulescens) ● Greater White-fronted Goose (Anser albifrons) ● Brant (Branta bernicla) ● Canada Goose (Branta canadensis) ● Wood Duck (Aix sponsa) ● Blue-winged Teal (Spatula discors) ● Northern Shoveler (Spatula clypeata) ● Gadwall (Mareca strepera) ● American Wigeon (Mareca americana) ● Mallard (Anas platyrhynchos) ● American Black Duck (Anas rubripes) ● Northern Pintail (Anas acuta) ● Green-winged Teal (Anas crecca) ● Ring-necked Duck (Aythya collaris) ● Greater Scaup (Aytha marila) ● Lesser Scaup (Aythya affinis) ● King Eider (Somateria spectabilis) ● Common Eider (Somateria mollissima) ● HarleQuin Duck (Histrionicus histrionicus) Threatened Species ME ● Surf Scoter (Melanitta perspicillata) ● White-winged Scoter (Melanitta fusca) ● Black Scoter (Melanitta americana) ● Long-tailed Duck (Clangula hyemalis) ● Bufflehead (Bucephala albeola) ● Common Goldeneye (Bucephala clangula) ● Barrow's Goldeneye (Bucephala islandica) Threatened Species ME ● Hooded Merganser (Lophodytes cucullatus) ● Common Merganser (Mergus merganser) ● Red-breasted Merganser (Mergus serrator) ● Ruddy Duck (Oxyura jamaicensis) Species not found in Maine (usually) but still eligible ● Black-bellied Whistling-Duck (Dendrocygna -

Spain - Realm of the Iberian Lynx
Spain - Realm of the Iberian Lynx Naturetrek Tour Report 19 - 24 January 2020 Iberian Lynx Iberian Lynx Cinereous Vulture Sunset Report compiled by Niki Williamson Images by Simon Tonkin Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report Spain - Realm of the Iberian Lynx Tour participants: Simon Tonkin & Niki Williamson (leaders) with 13 Naturetrek clients.. Summary For our band of patient nature-lovers, this six-day exploration of the realm of the Iberian Lynx gave us something amazing every day! Six different individuals of the endangered Spanish Imperial Eagle, rare Marbled Ducks, Hawfinches, Spanish Ibex cantering across a rock face, herds of Red Deer swimming a lake, duetting Little Owls, clouds of Cinereous and Griffon Vultures, Golden Eagles and shades of blue in the form of Bluethroat, Blue Rock Thrush, Iberian Magpie and Common Kingfisher were just some of our trip´s natural highlights. Our hosts´ hospitality was fantastic at both bases, and the group enjoyed sampling delicious local food such as chickpea and spinach stew, salmorejo soup and egg revuelto dishes, not to mention mouth-watering picnics in the sun, sometimes accompanied by dazzling flocks of Iberian Magpies, always accompanied by wine! Our fleeting glimpse of a female Iberian Lynx in Doñana Natural Park was to provide a suitable appetite-whetter for our superb encounter in Sierra de Morena, where a stunning female stalked across the track in front of us before taking up a pose on a nearby rock, allowing us to watch for over an hour! Day 1 Sunday 19th January Leaders Simon and Niki met the group as they converged on Sevilla airport, from various flights and pre-trip stays. -

Important Bird Areas and Potential Ramsar Sites in Europe
cover def. 25-09-2001 14:23 Pagina 1 BirdLife in Europe In Europe, the BirdLife International Partnership works in more than 40 countries. Important Bird Areas ALBANIA and potential Ramsar Sites ANDORRA AUSTRIA BELARUS in Europe BELGIUM BULGARIA CROATIA CZECH REPUBLIC DENMARK ESTONIA FAROE ISLANDS FINLAND FRANCE GERMANY GIBRALTAR GREECE HUNGARY ICELAND IRELAND ISRAEL ITALY LATVIA LIECHTENSTEIN LITHUANIA LUXEMBOURG MACEDONIA MALTA NETHERLANDS NORWAY POLAND PORTUGAL ROMANIA RUSSIA SLOVAKIA SLOVENIA SPAIN SWEDEN SWITZERLAND TURKEY UKRAINE UK The European IBA Programme is coordinated by the European Division of BirdLife International. For further information please contact: BirdLife International, Droevendaalsesteeg 3a, PO Box 127, 6700 AC Wageningen, The Netherlands Telephone: +31 317 47 88 31, Fax: +31 317 47 88 44, Email: [email protected], Internet: www.birdlife.org.uk This report has been produced with the support of: Printed on environmentally friendly paper What is BirdLife International? BirdLife International is a Partnership of non-governmental conservation organisations with a special focus on birds. The BirdLife Partnership works together on shared priorities, policies and programmes of conservation action, exchanging skills, achievements and information, and so growing in ability, authority and influence. Each Partner represents a unique geographic area or territory (most often a country). In addition to Partners, BirdLife has Representatives and a flexible system of Working Groups (including some bird Specialist Groups shared with Wetlands International and/or the Species Survival Commission (SSC) of the World Conservation Union (IUCN)), each with specific roles and responsibilities. I What is the purpose of BirdLife International? – Mission Statement The BirdLife International Partnership strives to conserve birds, their habitats and global biodiversity, working with people towards sustainability in the use of natural resources. -

Print 01/03 January 2003
A review of the status and identification of American Wigeon in Britain & Ireland Stephen C.Votier, Andrew H. J. Harrop and Matthew Denny John Wright ABSTRACT The numbers of American Wigeons Anas americana recorded in Britain & Ireland have increased significantly since the establishment of BBRC in 1958, and records ceased to be considered by the Committee from 1st January 2002.The status and distribution of the species is analysed here, and its identification discussed.Although male American Wigeon in breeding plumage is very distinctive, the identification of other plumages is much more problematic. ecords of American Wigeon Anas ameri- only were available). From 1st January 2002, cana, previously considered a rare records of American Wigeon ceased to be Rvagrant to Britain & Ireland from North assessed by BBRC, since the criteria for its America, have increased considerably since the removal from the list of species considered had mid 1980s, and there were 462 accepted records been met: more than 150 individuals had been by the end of 2001 (Rogers 2002; P. A. Fraser in recorded in the previous decade, with at least litt; note that Irish records until the end of 2000 ten in eight of those years. With this in mind, it 2 © British Birds 96 • January 2003 • 2-22 Status and identification of American Wigeon seems timely to document the status and distri- Status and distribution bution of the species, particularly given the American Wigeon breeds throughout northern problems of separating genuine vagrant wild- North America, from Alaska to Hudson Bay, fowl from escapes. In addition, although male and south through the Prairies to the eastern American Wigeon in breeding plumage is a rel- seaboard (Cramp & Simmons 1977; Madge & atively easy bird to identify, the identification of Burn 1988).