Sustainable Management of Migratory European Ducks: Finding Model Species Author(S): Sari Holopainen, Céline Arzel, Johan Elmberg, Anthony D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Information Sheet on Ramsar Wetlands (RIS)
Information Sheet on Ramsar Wetlands (RIS) Categories approved by Recommendation 4.7 (1990), as amended by Resolution VIII.13 of the 8th Conference of the Contracting Parties (2002) and Resolutions IX.1 Annex B, IX.6, IX.21 and IX. 22 of the 9th Conference of the Contracting Parties (2005). Notes for compilers: 1. The RIS should be completed in accordance with the attached Explanatory Notes and Guidelines for completing the Information Sheet on Ramsar Wetlands. Compilers are strongly advised to read this guidance before filling in the RIS. 2. Further information and guidance in support of Ramsar site designations are provided in the Strategic Framework for the future development of the List of Wetlands of International Importance (Ramsar Wise Use Handbook 7, 2nd edition, as amended by COP9 Resolution IX.1 Annex B). A 3rd edition of the Handbook, incorporating these amendments, is in preparation and will be available in 2006. 3. Once completed, the RIS (and accompanying map(s)) should be submitted to the Ramsar Secretariat. Compilers should provide an electronic (MS Word) copy of the RIS and, where possible, digital copies of all maps. 1. Name and address of the compiler of this form: FOR OFFICE USE ONLY. DD MM YY Joint Nature Conservation Committee Monkstone House City Road Designation date Site Reference Number Peterborough Cambridgeshire PE1 1JY UK Telephone/Fax: +44 (0)1733 – 562 626 / +44 (0)1733 – 555 948 Email: [email protected] 2. Date this sheet was completed/updated: Designated: 28 November 1985 3. Country: UK (England) 4. Name of the Ramsar site: Martin Mere 5. -

Rapid Risk Assessment on Incursion of HPAI H5N8 Into Housed Or Not Housed Poultry Flocks and Captive Birds
Rapid risk assessment on incursion of HPAI H5N8 into housed or not housed poultry flocks and captive birds 29 January 2021 Situation as at 26 January 2021 © Crown copyright 2021 You may re-use this information (excluding logos) free of charge in any format or medium, under the terms of the Open Government Licence v.3. To view this licence visit www.nationalarchives.gov.uk/doc/open-government-licence/version/3/ or email [email protected] This publication is available at www.gov.uk/government/publications Any enquiries regarding this publication should be sent to: [email protected] www.gov.uk/defra 2 Contents Summary ............................................................................................................................................. 4 Introduction ........................................................................................................................................ 6 Hazard Identification ......................................................................................................................... 10 Previous outbreaks of HPAI H5N8: ................................................................................................... 12 Current Situation ............................................................................................................................... 12 Risk Question .................................................................................................................................... 16 Risk Levels .................................................................................................................................... -

Red-Breasted Goose
Urgent preliminary assessment of ornithological data relevant to spread of Avian Influenza in Europe Ward Hagemeijer Wetlands International Commissioned by DG Environment to Wetlands International and Euring Data needs for risk assessment: ornithology (virology) Quantified risk assessment: Research and Monitoring programme A. Birds as a vector 1.Quantified bird migration information: satellite telemetry, ringing data analysis, count data analysis 2.Quantified frequency of occurrence of virus in wild birds: surveillance 3.Ecology of virus in wild birds: virological research on wild birds B. Impact on wild bird populations Different components of the project Activities to be undertaken: • identification of Higher Risk Species (HRS) • analysis of their migration routes (ringing and count data) • identification of concentration and mixing sites • rapid assessment planning for wetland sites Analysis of higher risk species Identification of HRS on basis of: • occurence of LPAI viruses • ecology and behaviour • contact risk with poultry • numbers within EU In collaboration with David Stroud and Rowena Langston Occurrence of LPAI in wild birds species Results 1999 – 2004 Erasmus University Netherlands Species N Tested N PCR+ (%) N Egg + Mallard 6822 489 (7.2) 267 Eurasian Wigeon 1470 22 (1.5) 4 Common Teal 670 18 (2.7) 4 Northern Pintail 135 4 (3.0) 1 Northern Shoveler 90 1 (1.1) 1 Shelduck, Eider, Gadwall, Tufted, Garganey 238 0 (0.0) 0 Greater White-fronted Goose 1696 19 (1.1) 5 Greylag Goose 303 8 (2.6) 4 Brent, Barnacle, Bean, Egyptian, Canada, Pink-f 1202 0 (0.0) 0 Black-headed Gull 993 10 (1.0) 6 Common, Herring, Black-backed, Kittiwake 1976 0 (0.0) 0 Guillemot 698 3 (0.4) 1 Other birds 10909 0 0 + + + 27204 574 (2.1) 295 Selection of taxonomic groups • Anseriformes and Charadriiformes, • Migratory • Occurring in Europe. -

THE FAMILY ANATIDAE 43 Ernst Map
March 1945 42 THE WILSON BULLETIN Vol. 57, No. 1 Biziura L lob&, Australian Musk Duck Aberrant Species Thalassornis leuconota, African White-backed Duck Heteronetta atricapilla, Black-headed Duck 7. TRIBE MERGANETTINI. TORRENTDUCKS Merganetta armuta, Torrent Duck GENERA RECOGNIZEDBY PETERS AND SYNONYMIZEDIHERE Arctonetta= Som&eria Metopiana= Netta Asarcornis = Cairina Nesochen= Branta Casarca = Tadorna Nesonetta= Anus Chaulelasmus= Anas Nomonyx = Oxyura Chen = Anser Nyroca= Aythya Cheniscus= Nettapus Oidemia = Melanitta Chenopis= Cygnus Phil&e= Anser Cygnopsis= Anser Polysticta= Somateria Dendronessa= Aix Pseudotadorna= Tadorna Eulabeia= Anser Pteronetta= Cairina Lophodytes= Mergus Salvadorina= Anus Mareca= Anas Spatula= Anas Mergellus = Mergus GENERA RECOGNIZEDHERE BUT NOT BY PETERS Amazonetta von Boetticher (for Anus brasiliensis) Lophonetta Riley (for Anus specularioides) COMPARISONOP CHARACTERS Our studies have shown that the waterfowl can be divided into about nine groups that are fairly well defined both morphologically and biologically. In addition, there are a number of species and genera that are either intermediate between the otherwise well- defined tribes (e.g. Coscoroba) or too poorly known for a safe classi- fication (e.g. Anus specularis, Anus leucophrys, Malacorhynchus, Tachyeres) ; others show peculiarities or a combination of characters that prevent them from fitting well into any of the existing groups. Such genera as the Australian Cereopsis, Anseranas, Stictonetta, and Chenonetta could either be made the sole representatives of so many separate tribes or each could be included in the tribe with which it shares the greatest number of similarities. For the sake of con- venience we have adopted the latter course, but without forgetting that these genera are not typical representatives of the tribes with which we associate them. -

Waterfowl in Iowa, Overview
STATE OF IOWA 1977 WATERFOWL IN IOWA By JACK W MUSGROVE Director DIVISION OF MUSEUM AND ARCHIVES STATE HISTORICAL DEPARTMENT and MARY R MUSGROVE Illustrated by MAYNARD F REECE Printed for STATE CONSERVATION COMMISSION DES MOINES, IOWA Copyright 1943 Copyright 1947 Copyright 1953 Copyright 1961 Copyright 1977 Published by the STATE OF IOWA Des Moines Fifth Edition FOREWORD Since the origin of man the migratory flight of waterfowl has fired his imagination. Undoubtedly the hungry caveman, as he watched wave after wave of ducks and geese pass overhead, felt a thrill, and his dull brain questioned, “Whither and why?” The same age - old attraction each spring and fall turns thousands of faces skyward when flocks of Canada geese fly over. In historic times Iowa was the nesting ground of countless flocks of ducks, geese, and swans. Much of the marshland that was their home has been tiled and has disappeared under the corn planter. However, this state is still the summer home of many species, and restoration of various areas is annually increasing the number. Iowa is more important as a cafeteria for the ducks on their semiannual flights than as a nesting ground, and multitudes of them stop in this state to feed and grow fat on waste grain. The interest in waterfowl may be observed each spring during the blue and snow goose flight along the Missouri River, where thousands of spectators gather to watch the flight. There are many bird study clubs in the state with large memberships, as well as hundreds of unaffiliated ornithologists who spend much of their leisure time observing birds. -
Greater White-Fronted Goose
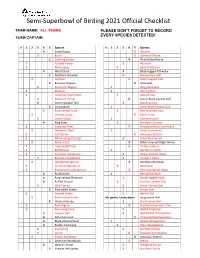
Semi-Superbowl of Birding 2021 Official Checklist TEAM NAME: ALL TEAMS PLEASE DON’T FORGET TO RECORD EVERY SPECIES DETECTED! TEAM CAPTAIN: √ 1 2 3 4 5 Species √ 1 2 3 4 5 Species 4 Snow Goose 5 Dovekie 3 Brant 5 Common Murre 5 Cackling Goose 4 Thick-billed Murre 1 Canada Goose 3 Razorbill 1 Mute Swan 2 Black Guillemot 4 Wood Duck 3 Black-legged Kittiwake 5 Northern Shoveler 3 Bonaparte’s Gull 2 Gadwall 4 Black-headed Gull 5 Eurasian Wigeon 5 Little Gull 3 American Wigeon 1 Ring-billed Gull 1 Mallard 1 Herring Gull 1 American Black Duck 2 Iceland Gull 3 Northern Pintail 4 Lesser Black-backed Gull 3 Green-winged Teal 3 Glaucous Gull 5 Canvasback 1 Great Black-backed Gull 4 Ring-necked Duck 2 Red-throated Loon 2 Greater Scaup 5 Pacific Loon 3 Lesser Scaup 1 Common Loon 4 King Eider 2 Northern Gannet 1 Common Eider 4 Double-crested Cormorant 2 Harlequin Duck 1 Great Cormorant 1 Surf Scoter 5 American Bittern 1 White-winged Scoter 3 Great Blue Heron 2 Black Scoter 4 Black-crowned Night-Heron 1 Long-tailed Duck 4 Turkey Vulture 1 Bufflehead 1 Northern Harrier 1 Common Goldeneye 3 Sharp-shinned Hawk 3 Barrow's Goldeneye 3 Cooper’s Hawk 2 Hooded Merganser 4 Northern Goshawk 1 Common Merganser 2 Bald Eagle 1 Red-breasted Merganser 4 Red-shouldered Hawk 4 Ruddy Duck 1 Red-tailed Hawk 4 Ring-necked Pheasant 3 Rough-legged hawk 4 Ruffed Grouse 2 Eastern Screech-Owl 3 Wild Turkey 3 Great Horned Owl 5 Pied-billed Grebe 3 Snowy Owl 1 Horned Grebe 3 Barred Owl 2 Red-necked Grebe No points. -

European Red List of Birds 2015
Mareca strepera (Gadwall) European Red List of Birds Supplementary Material The European Union (EU27) Red List assessments were based principally on the official data reported by EU Member States to the European Commission under Article 12 of the Birds Directive in 2013-14. For the European Red List assessments, similar data were sourced from BirdLife Partners and other collaborating experts in other European countries and territories. For more information, see BirdLife International (2015). Contents Reported national population sizes and trends p. 2 Trend maps of reported national population data p. 6 Sources of reported national population data p. 9 Species factsheet bibliography p. 17 Recommended citation BirdLife International (2015) European Red List of Birds. Luxembourg: Office for Official Publications of the European Communities. Further information http://www.birdlife.org/datazone/info/euroredlist http://www.birdlife.org/europe-and-central-asia/european-red-list-birds-0 http://www.iucnredlist.org/initiatives/europe http://ec.europa.eu/environment/nature/conservation/species/redlist/ Data requests and feedback To request access to these data in electronic format, provide new information, correct any errors or provide feedback, please email [email protected]. THE IUCN RED LIST OF THREATENED SPECIES™ BirdLife International (2015) European Red List of Birds Mareca strepera (Gadwall) Table 1. Reported national breeding population size and trends in Europe1. Country (or Population estimate Short-term population trend4 Long-term population trend4 Subspecific population (where relevant) 2 territory) Size (pairs)3 Europe (%) Year(s) Quality Direction5 Magnitude (%)6 Year(s) Quality Direction5 Magnitude (%)6 Year(s) Quality Albania 0-5 <1 2002-2012 poor ? ? Armenia 250-400 <1 2002-2012 medium ? ? Austria 250-350 <1 2001-2012 good 0 0 2001-2012 medium + 50-100 1980-2012 medium M. -

2020 MIGRATORY GAME BIRD SEASON PREVIEW Summary of Issues for Consideration
Comments may be sent to [email protected] 2020 MIGRATORY GAME BIRD SEASON PREVIEW Summary of Issues for Consideration: The majority of Vermont’s waterfowl season is driven by the federal framework for the Atlantic Flyway. Below are a few issues that must be decided for the 2020 hunting season. The Department would like the Board to consider the following: • Hold the liberal season allowed under the federal framework related to season lengths and daily bag limits. The Board has the option to be more conservative. • For the 2020 Duck Season. o Open the 2020 duck season on a Saturday, October 10. The change to opening every other year on a Saturday and Wednesday is consistent with hunter preferences of moving to alternating year approach for openings. o Any splits within seasons to create segments should be considered for the Lake Champlain and Interior Vermont zones. o Interior Zone: October 10 and run through December 8. o Lake Champlain Zone: October 10 - Nov. 1 and Nov. 21 - Dec. 27. • For the 2020 Goose Seasons o Open the resident Canada goose season September 1st and continue through September 25. o Open the migratory Canada goose season on October 10. o Opening the Snow goose season on October 1. • Hold youth hunting weekend – September 26-27. • Hold woodcock/snipe season: October 1- November 14. • Board has option to have a two-day active duty/veterans hunt. Currently, the Department does not propose doing this. Background In 2016 the Department began fully reviewing the migratory game bird season options with the Board without being under a very short time constraint. -

Texas Wildlife Identification Guide: a Guide to Game Animals, Game
texas parks and wildlife TEXAS WILDLIFE IDENTIFICATION GUIDE A guide to game animals, game birds, furbearers and other wildlife of Texas. INTRODUCTION TEXAS game animals, game birds, furbearers and other wildlife are important for many reasons. They provide countless hours of viewing and recreational opportunities.They benefit the Texas economy through hunting and “nature tourism” such as birdwatching. Commercial businesses that provide birdseed, dry corn and native landscaping may be devoted solely to attracting many of the animals found in this book. Local hunting and trapping economies, guiding operations and hunting leases have prospered because of the abun- dance of these animals in Texas.The Texas Parks and Wildlife Department benefits because of hunting license sales, but it uses these funds to research, manage and pro- tect all wildlife populations – not just game animals. Game animals provide humans with cultural, social, aesthetic and spiritual pleasures found in wildlife art, taxi- dermy and historical artifacts. Conservation organizations dedicated to individual species such as quail, turkey and deer, have funded thousands of wildlife projects throughout North America, demonstrating the mystique game animals have on people. Animals referenced in this pocket guide exist because their habitat exists in Texas. Habitat is food, cover, water and space, all suitably arranged.They are part of a vast food chain or web that includes thousands more species of wildlife such as the insects, non-game animals, fish and i rare/endangered species. Active management of wild landscapes is the primary means to continue having abundant populations of wildlife in Texas. Preservation of rare and endangered habitat is one way of saving some species of wildlife such as the migratory whooping crane that makes Texas its home in the winter. -

Nest Box Guide for Waterfowl Nest Box Guide for Waterfowl Copyright © 2008 Ducks Unlimited Canada ISBN 978-0-9692943-8-2
Nest Box Guide for Waterfowl Nest Box Guide For Waterfowl Copyright © 2008 Ducks Unlimited Canada ISBN 978-0-9692943-8-2 Any reproduction of this present document in any form is illegal without the written authorization of Ducks Unlimited Canada. For additional copies please contact the Edmonton DUC office at (780)489-2002. Published by: Ducks Unlimited Canada www.ducks.ca Acknowledgements Photography provided by : Ducks Unlimited Canada (DUC), Jim Potter (Alberta Conservation Association (ACA)), Darwin Chambers (DUC), Jonathan Thompson (DUC), Lesley Peterson (DUC contractor), Sherry Feser (ACA), Gordon Court ( p 16 photo of Pygmy Owl), Myrna Pearman ,(Ellis Bird Farm), Bryan Shantz and Glen Rowan. Portions of this booklet are based on a Nest Box Factsheet prepared by Jim Potter (ACA) and Lesley Peterson (DUC contractor). Myrna Pearman provided editorial comment. Table of Contents Table of Contents Why Nest Boxes? ......................................................................................................1 Natural Cavities ......................................................................................................................................2 Identifying Wildlife Species That Use Your Nest Boxes .....................................3 Waterfowl ..................................................................................................................4 Common Goldeneye .........................................................................................................................5 Barrow’s Goldeneye -

Handbook of Waterfowl Behavior: Tribe Anatini (Surface-Feeding Ducks)
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Handbook of Waterfowl Behavior, by Paul Johnsgard Papers in the Biological Sciences January 1965 Handbook of Waterfowl Behavior: Tribe Anatini (Surface-feeding Ducks) Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/bioscihandwaterfowl Part of the Ornithology Commons Johnsgard, Paul A., "Handbook of Waterfowl Behavior: Tribe Anatini (Surface-feeding Ducks)" (1965). Handbook of Waterfowl Behavior, by Paul Johnsgard. 16. https://digitalcommons.unl.edu/bioscihandwaterfowl/16 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Handbook of Waterfowl Behavior, by Paul Johnsgard by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Subfamily Anatinae 125 Aix. During extreme excitement the male will often roll his head on his back, or even bathe. I have not observed Preening-behind-the- wing, but W. von de Wall (pers. comm.) has observed a male per- form it toward a female. Finally, Wing-flapping appears to be used as a display by males, and it is especially conspicuous because each sequence of it is ended by a rapid stretching of both wings over the back in a posture that makes visible the white axillary feathers, which contrast sharply with the black underwing surface. Copulatory behavior. Precopulatory behavior consists of the male swimming up to the female, his neck stretched and his crest de- pressed, and making occasional Bill-dipping movements. He then suddenly begins to perform more vigorous Head-dipping movements, and the female, if receptive, performs similar Bill-dipping or Head- dipping movements. -

Derivation of Non-Breeding Duck
North American Waterfowl Management Plan Science Support Team Technical Report No. 2019–01 Derivation of Regional, Non-breeding Duck Population Abundance Objectives to Inform Conservation Planning in North America — 2019 Revision Kathy K. Fleming U.S. Fish and Wildlife Service, Division of Migratory Bird Management Michael K. Mitchell Ducks Unlimited, Inc. Southern Regional Office Michael G. Brasher Ducks Unlimited, Inc., Gulf Coast Joint Venture John M. Coluccy Ducks Unlimited, Inc., Great Lakes and Atlantic Regional Office J. Dale James Ducks Unlimited, Inc. Southern Regional Office Mark J. Petrie Ducks Unlimited, Inc., Western Regional Office, Pacific Coast Joint Venture Dale D. Humburg Ducks Unlimited, Inc., National Headquarters Gregory J. Soulliere U. S. Fish and Wildlife Service, Upper Mississippi / Great Lakes Joint Venture ABSTRACT During the early 2000s, a methodology was developed to derive regional non-breeding population abundance objectives from continental abundance estimates (M. Koneff, USFWS, unpublished data). This information was foundational to North American Waterfowl Management Plan (NAWMP) Joint Venture (JV) habitat conservation planning and implementation for non-breeding waterfowl, especially wintering ducks. The 2012 NAWMP Revision and its amended population objectives motivated JVs to begin updating their waterfowl implementation plans. Fleming et al. (2017) revisited the initial work to derive non- breeding abundance objectives and developed an updated approach. Although Fleming et al. (2017) made use of the least biased and most geographically consistent datasets, they identified outstanding issues to be resolved before the derivation technique could be effectively applied across all regions of North America. We updated the work of Fleming et al. (2017) by addressing 3 of those issues.