Printer Emulator for Testing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Earliest Interior Occurrence of Eurasian Wigeon (Anas Penelope) in British Columbia
Wildlife Afield 10(1):38-39, 2013 © Biodiversity Centre for Wildlife Studies Earliest Interior Occurrence of Eurasian Wigeon (Anas penelope) in British Columbia R. Wayne Campbell1 and Glenn R. Ryder2 12511 Kilgary Place, Victoria, British Columbia, Canada V8N 1J6 22302 ‒ 2888 273rd Street, Aldergrove, British Columbia, Canada V4W 3M6 From the late 19th century through the mid-20th through 9 January 1946 (Munro and Cowan 1947). century, Eurasian Wigeon (Anas penelope; Figure The species was reported during this period between 9 1) was considered an “Occasional visitant to coast January and 30 March (10 records) and on 30 October waters” (Brooks and Swarth 1925:32), becoming and 5 December. Ten of the records (75%) occurred a “Regular winter visitant to the coast” of British in January and February (Munro and Cowan 1947), Columbia (Munro and Cowan 1947:63). The latter the height of the winter season. Surprisingly, most status was based on 12 records of males collected occurrences were from southeastern Vancouver Island or sighted during the 48-year period from the first (75%) and not the adjacent southwest mainland coast provincial record in February 1898 (Kermode 1904) where huge flocks of American Wigeon (A. americana) Figure 1. Almost all records of Eurasian Wigeon in British Columbia are of males, as the females of this and the congeneric American Wigeon are difficult to separate in the field. One of the earliest provincial records was from Esquimalt Lagoon on 25 February 1934 where this male was photographed there 71 years later on 19 February 2005. Photo by Mark Nyhof 10:1 June 2013 38 overwinter (Campbell et al. -

Rapid Risk Assessment on Incursion of HPAI H5N8 Into Housed Or Not Housed Poultry Flocks and Captive Birds
Rapid risk assessment on incursion of HPAI H5N8 into housed or not housed poultry flocks and captive birds 29 January 2021 Situation as at 26 January 2021 © Crown copyright 2021 You may re-use this information (excluding logos) free of charge in any format or medium, under the terms of the Open Government Licence v.3. To view this licence visit www.nationalarchives.gov.uk/doc/open-government-licence/version/3/ or email [email protected] This publication is available at www.gov.uk/government/publications Any enquiries regarding this publication should be sent to: [email protected] www.gov.uk/defra 2 Contents Summary ............................................................................................................................................. 4 Introduction ........................................................................................................................................ 6 Hazard Identification ......................................................................................................................... 10 Previous outbreaks of HPAI H5N8: ................................................................................................... 12 Current Situation ............................................................................................................................... 12 Risk Question .................................................................................................................................... 16 Risk Levels .................................................................................................................................... -

Red-Breasted Goose
Urgent preliminary assessment of ornithological data relevant to spread of Avian Influenza in Europe Ward Hagemeijer Wetlands International Commissioned by DG Environment to Wetlands International and Euring Data needs for risk assessment: ornithology (virology) Quantified risk assessment: Research and Monitoring programme A. Birds as a vector 1.Quantified bird migration information: satellite telemetry, ringing data analysis, count data analysis 2.Quantified frequency of occurrence of virus in wild birds: surveillance 3.Ecology of virus in wild birds: virological research on wild birds B. Impact on wild bird populations Different components of the project Activities to be undertaken: • identification of Higher Risk Species (HRS) • analysis of their migration routes (ringing and count data) • identification of concentration and mixing sites • rapid assessment planning for wetland sites Analysis of higher risk species Identification of HRS on basis of: • occurence of LPAI viruses • ecology and behaviour • contact risk with poultry • numbers within EU In collaboration with David Stroud and Rowena Langston Occurrence of LPAI in wild birds species Results 1999 – 2004 Erasmus University Netherlands Species N Tested N PCR+ (%) N Egg + Mallard 6822 489 (7.2) 267 Eurasian Wigeon 1470 22 (1.5) 4 Common Teal 670 18 (2.7) 4 Northern Pintail 135 4 (3.0) 1 Northern Shoveler 90 1 (1.1) 1 Shelduck, Eider, Gadwall, Tufted, Garganey 238 0 (0.0) 0 Greater White-fronted Goose 1696 19 (1.1) 5 Greylag Goose 303 8 (2.6) 4 Brent, Barnacle, Bean, Egyptian, Canada, Pink-f 1202 0 (0.0) 0 Black-headed Gull 993 10 (1.0) 6 Common, Herring, Black-backed, Kittiwake 1976 0 (0.0) 0 Guillemot 698 3 (0.4) 1 Other birds 10909 0 0 + + + 27204 574 (2.1) 295 Selection of taxonomic groups • Anseriformes and Charadriiformes, • Migratory • Occurring in Europe. -

Waterfowl Management in Georgia
WATERFOWL MANAGEMENT IN GEORGIA PREFACE & ACKNOWLEDGMENTS Wildlife biologists serving on the Georgia Department of Natural Resources' Waterfowl Committee prepared the information found here. It is intended to serve as a source of general information for those with a casual interest in waterfowl. It also serves as a more detailed guide for landowners and managers who want to improve the waterfowl habitat on their property. The committee hopes this information will serve to benefit the waterfowl resource in Georgia and help to ensure its well- being for generations to come. Land management assistance is available from Wildlife Resources Division biologists. For additional help, contact the nearest Game Management Section office. Game Management Offices Region I Armuchee (706) 295-6041 Region II Gainesville (770) 535-5700 Region III Thomson (706) 595-4222 Region III Thomson (Augusta) (706) 667-4672 Region IV Fort Valley (478) 825-6354 Region V Albany (229) 430-4254 Region VI Fitzgerald (229) 426-5267 Region VII Brunswick (912) 262-3173 * Headquarters (770) 918-6416 We would like to express our appreciation to Carroll Allen and Dan Forster of the Georgia Department of Natural Resources for providing editorial comments. AUTHORS: Greg Balkcom, Senior Wildlife Biologist Ted Touchstone, Wildlife Biologist Kent Kammermeyer, Senior Wildlife Biologist Vic Vansant, Regional Wildlife Supervisor Carmen Martin, Wildlife Biologist Mike Van Brackle, Wildlife Biologist George Steele, Wildlife Biologist John Bowers, Senior Wildlife Biologist The Department of Natural Resources is an equal opportunity employer and offers all persons the opportunity to compete and participate in areas of employment regardless of race, color, religion, national origin, handicap, or other non-merit factors. -

Waterfowl in Iowa, Overview
STATE OF IOWA 1977 WATERFOWL IN IOWA By JACK W MUSGROVE Director DIVISION OF MUSEUM AND ARCHIVES STATE HISTORICAL DEPARTMENT and MARY R MUSGROVE Illustrated by MAYNARD F REECE Printed for STATE CONSERVATION COMMISSION DES MOINES, IOWA Copyright 1943 Copyright 1947 Copyright 1953 Copyright 1961 Copyright 1977 Published by the STATE OF IOWA Des Moines Fifth Edition FOREWORD Since the origin of man the migratory flight of waterfowl has fired his imagination. Undoubtedly the hungry caveman, as he watched wave after wave of ducks and geese pass overhead, felt a thrill, and his dull brain questioned, “Whither and why?” The same age - old attraction each spring and fall turns thousands of faces skyward when flocks of Canada geese fly over. In historic times Iowa was the nesting ground of countless flocks of ducks, geese, and swans. Much of the marshland that was their home has been tiled and has disappeared under the corn planter. However, this state is still the summer home of many species, and restoration of various areas is annually increasing the number. Iowa is more important as a cafeteria for the ducks on their semiannual flights than as a nesting ground, and multitudes of them stop in this state to feed and grow fat on waste grain. The interest in waterfowl may be observed each spring during the blue and snow goose flight along the Missouri River, where thousands of spectators gather to watch the flight. There are many bird study clubs in the state with large memberships, as well as hundreds of unaffiliated ornithologists who spend much of their leisure time observing birds. -
Greater White-Fronted Goose
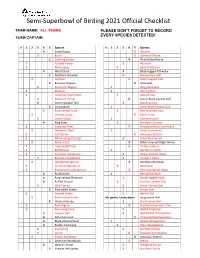
Semi-Superbowl of Birding 2021 Official Checklist TEAM NAME: ALL TEAMS PLEASE DON’T FORGET TO RECORD EVERY SPECIES DETECTED! TEAM CAPTAIN: √ 1 2 3 4 5 Species √ 1 2 3 4 5 Species 4 Snow Goose 5 Dovekie 3 Brant 5 Common Murre 5 Cackling Goose 4 Thick-billed Murre 1 Canada Goose 3 Razorbill 1 Mute Swan 2 Black Guillemot 4 Wood Duck 3 Black-legged Kittiwake 5 Northern Shoveler 3 Bonaparte’s Gull 2 Gadwall 4 Black-headed Gull 5 Eurasian Wigeon 5 Little Gull 3 American Wigeon 1 Ring-billed Gull 1 Mallard 1 Herring Gull 1 American Black Duck 2 Iceland Gull 3 Northern Pintail 4 Lesser Black-backed Gull 3 Green-winged Teal 3 Glaucous Gull 5 Canvasback 1 Great Black-backed Gull 4 Ring-necked Duck 2 Red-throated Loon 2 Greater Scaup 5 Pacific Loon 3 Lesser Scaup 1 Common Loon 4 King Eider 2 Northern Gannet 1 Common Eider 4 Double-crested Cormorant 2 Harlequin Duck 1 Great Cormorant 1 Surf Scoter 5 American Bittern 1 White-winged Scoter 3 Great Blue Heron 2 Black Scoter 4 Black-crowned Night-Heron 1 Long-tailed Duck 4 Turkey Vulture 1 Bufflehead 1 Northern Harrier 1 Common Goldeneye 3 Sharp-shinned Hawk 3 Barrow's Goldeneye 3 Cooper’s Hawk 2 Hooded Merganser 4 Northern Goshawk 1 Common Merganser 2 Bald Eagle 1 Red-breasted Merganser 4 Red-shouldered Hawk 4 Ruddy Duck 1 Red-tailed Hawk 4 Ring-necked Pheasant 3 Rough-legged hawk 4 Ruffed Grouse 2 Eastern Screech-Owl 3 Wild Turkey 3 Great Horned Owl 5 Pied-billed Grebe 3 Snowy Owl 1 Horned Grebe 3 Barred Owl 2 Red-necked Grebe No points. -

2019 Waterfowl Population Status Survey
U.S. Fish & Wildlife Service Waterfowl Population Status, 2019 Waterfowl Population Status, 2019 August 19, 2019 In the United States the process of establishing hunting regulations for waterfowl is conducted annually. This process involves a number of scheduled meetings in which information regarding the status of waterfowl is presented to individuals within the agencies responsible for setting hunting regulations. In addition, the proposed regulations are published in the Federal Register to allow public comment. This report includes the most current breeding population and production information available for waterfowl in North America and is a result of cooperative eforts by the U.S. Fish and Wildlife Service (USFWS), the Canadian Wildlife Service (CWS), various state and provincial conservation agencies, and private conservation organizations. In addition to providing current information on the status of populations, this report is intended to aid the development of waterfowl harvest regulations in the United States for the 2020–2021 hunting season. i Acknowledgments Waterfowl Population and Habitat Information: The information contained in this report is the result of the eforts of numerous individuals and organizations. Principal contributors include the Canadian Wildlife Service, U.S. Fish and Wildlife Service, state wildlife conservation agencies, provincial conservation agencies from Canada, and Direcci´on General de Conservaci´on Ecol´ogica de los Recursos Naturales, Mexico. In addition, several conservation organizations, other state and federal agencies, universities, and private individuals provided information or cooperated in survey activities. Appendix A.1 provides a list of individuals responsible for the collection and compilation of data for the “Status of Ducks” section of this report. -

HUNTING-SEASONS.Pdf
HUNTING SEASON AUGUST 1 - JULY 31 AUG SEP OCT NOV DEC JAN FEB MAR APR MAY JUN JUL White tailed deer (Odocoileus virginianus) Open season Sep 1 - Feb 15 Roedeer (Capreolus capreolus) Open season Sep 1 - Feb 15 Roe buck May 5 - Jun 15 Moose (alces alces) Open season Sep 1 - Jan 15 Fallow deer (Dama dama) Open season Sep 1 - Jan 31 Mouflon (Ovis orientalis musimon) Open season Sep 1 - Jan 31 Wild boar (Sus scrofa) Open season all year round. Females with piglets are protected Mar 1 - Jul 31 Caribou (Rangifer tarandus) Open season Sep 30 - Jan 31 Brown hare (Lepus europaeus) Open season Sep 1 - Feb 28 Mountain hare (Lepus timidus) Open season Sep 1 - Feb 28 Rabbit (Oryctolagus cuniculus) Open season Sep 1 - Feb 28 European beaver (Castor fiber) Open season Aug 20 - Apr 30 North American beaver (Castor canadensis) Open season Aug 20 - Apr 30 Muskrat (Ondatra zibethicus) Open season Oct 1 - May 19 Red squirrel (Sciurus vulgaris) Nov 1-Feb 31 Brown bear (Ursus arctos) Open season Aug 20 - Oct 31 Grey wolf (Canis lupus) Lynx (Lynx lynx) Dec 1 - Feb 28 Wolverine (Gulo gulo) Grey seal (Halichoerus grypus) Open season Apr 16 - Dec 31 Open season Apr 16 - Dec 31 Ringed seal (Pusa hispida botnica) Open season Apr 16 - Dec 31 Open season Apr 16 - Dec 31 Red fox (Vulpes vulpes) Open season Aug 1 - Apr 14 Raccoon dog (Nyctereutes procyonoides) Open season all year round. Females with cubs are protected between May 1 - July 31 Badger (Meles meles) Open season Aug 1 - Mar 31 American Mink (Neovison vison) Open season all year round. -

(2007): Birds of the Aleutian Islands, Alaska Please
Bold* = Breeding Sp Su Fa Wi Bold* = Breeding Sp Su Fa Wi OSPREYS FINCHES Osprey Ca Ca Ac Brambling I Ca Ca EAGLES and HAWKS Hawfinch I Ca Northern Harrier I I I Common Rosefinch Ca Eurasian Sparrowhawk Ac (Ac) Pine Grosbeak Ca Bald Eagle* C C C C Asian Rosy-Finch Ac Rough-legged Hawk Ac Ca Ca Gray-crowned Rosy-Finch* C C C C OWLS (griseonucha) Snowy Owl I Ca I I Gray-crowned Rosy-Finch (littoralis) Ac Short-eared Owl* R R R U Oriental Greenfinch Ca FALCONS Common Redpoll I Ca I I Eurasian Kestrel Ac Ac Hoary Redpoll Ca Ac Ca Ca Merlin Ca I Red Crossbill Ac Gyrfalcon* R R R R White-winged Crossbill Ac Peregrine Falcon* (pealei) U U C U Pine Siskin I Ac I SHRIKES LONGSPURS and SNOW BUNTINGS Northern Shrike Ca Ca Ca Lapland Longspur* Ac-C C C-Ac Ac CROWS and JAYS Snow Bunting* C C C C Common Raven* C C C C McKay's Bunting Ca Ac LARKS EMBERIZIDS Sky Lark Ca Ac Rustic Bunting Ca Ca SWALLOWS American Tree Sparrow Ac Tree Swallow Ca Ca Ac Savannah Sparrow Ca Ca Ca Bank Swallow Ac Ca Ca Song Sparrow* C C C C Cliff Swallow Ca Golden-crowned Sparrow Ac Ac Barn Swallow Ca Dark-eyed Junco Ac WRENS BLACKBIRDS Pacific Wren* C C C U Rusty Blackbird Ac LEAF WARBLERS WOOD-WARBLERS Bold* = Breeding Sp Su Fa Wi Wood Warbler Ac Yellow Warbler Ac Dusky Warbler Ac Blackpoll Warbler Ac DUCKS, GEESE and SWANS Kamchatka Leaf Warbler Ac Yellow-rumped Warbler Ac Emperor Goose C-I Ca I-C C OLD WORLD FLYCATCHERS "HYPOTHETICAL" species needing more documentation Snow Goose Ac Ac Gray-streaked Flycatcher Ca American Golden-plover (Ac) Greater White-fronted Goose I -

American Wigeon Anas Americana
Migratory Birds American Wigeon Anas americana SPECIES STATUS: State recognized as Indigenous Photo: Eric VanderWerf SPECIES INFORMATION: The American wigeon, or "Baldpate," is a widespread North American dabbling duck (Family: Anatidae), which winters in small numbers in the Hawaiian Islands. Wigeons are common across most of North America, with a breeding population estimated in excess of three million. While wigeons are dun-colored across most of the body, breeding males sport a bright white stripe across the crown of the head, bright green patches on either side of the head, and contrasting white and dark plumage on the rump. During the breeding season, males' contrasting colors are used in courtship displays to attract females and discourage competing males. However, in winter, male plumage is much more similar to that of females, retaining the brown body coloration but changing to dark gray on the head and neck. Wigeons are the most vegetarian of dabbling ducks, eating the stems and leafy parts of aquatic plants, leafy parts of grasses, and leaves and seeds of some marsh and crop plants. Breeding females, however, feed largely on insects (including dragonflies and damselflies), mollusks, and crustaceans. DISTRIBUTION: In North America, wigeons' winter range extends across the central and southern United States, south through Mexico, and along the U.S. east coast as far north as Cape Cod. In Hawai‘i, they have been sighted throughout the Main Hawaiian Islands (MHI) but have not been recorded in the Northwestern Hawaiian Islands. ABUNDANCE: U.S. Fish and Wildlife Service surveys from 1955 through 1997 yield an average breeding population estimate of approximately 2.62 million birds for North America. -

Spain - Realm of the Iberian Lynx
Spain - Realm of the Iberian Lynx Naturetrek Tour Report 19 - 24 January 2020 Iberian Lynx Iberian Lynx Cinereous Vulture Sunset Report compiled by Niki Williamson Images by Simon Tonkin Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report Spain - Realm of the Iberian Lynx Tour participants: Simon Tonkin & Niki Williamson (leaders) with 13 Naturetrek clients.. Summary For our band of patient nature-lovers, this six-day exploration of the realm of the Iberian Lynx gave us something amazing every day! Six different individuals of the endangered Spanish Imperial Eagle, rare Marbled Ducks, Hawfinches, Spanish Ibex cantering across a rock face, herds of Red Deer swimming a lake, duetting Little Owls, clouds of Cinereous and Griffon Vultures, Golden Eagles and shades of blue in the form of Bluethroat, Blue Rock Thrush, Iberian Magpie and Common Kingfisher were just some of our trip´s natural highlights. Our hosts´ hospitality was fantastic at both bases, and the group enjoyed sampling delicious local food such as chickpea and spinach stew, salmorejo soup and egg revuelto dishes, not to mention mouth-watering picnics in the sun, sometimes accompanied by dazzling flocks of Iberian Magpies, always accompanied by wine! Our fleeting glimpse of a female Iberian Lynx in Doñana Natural Park was to provide a suitable appetite-whetter for our superb encounter in Sierra de Morena, where a stunning female stalked across the track in front of us before taking up a pose on a nearby rock, allowing us to watch for over an hour! Day 1 Sunday 19th January Leaders Simon and Niki met the group as they converged on Sevilla airport, from various flights and pre-trip stays. -

Print 01/03 January 2003
A review of the status and identification of American Wigeon in Britain & Ireland Stephen C.Votier, Andrew H. J. Harrop and Matthew Denny John Wright ABSTRACT The numbers of American Wigeons Anas americana recorded in Britain & Ireland have increased significantly since the establishment of BBRC in 1958, and records ceased to be considered by the Committee from 1st January 2002.The status and distribution of the species is analysed here, and its identification discussed.Although male American Wigeon in breeding plumage is very distinctive, the identification of other plumages is much more problematic. ecords of American Wigeon Anas ameri- only were available). From 1st January 2002, cana, previously considered a rare records of American Wigeon ceased to be Rvagrant to Britain & Ireland from North assessed by BBRC, since the criteria for its America, have increased considerably since the removal from the list of species considered had mid 1980s, and there were 462 accepted records been met: more than 150 individuals had been by the end of 2001 (Rogers 2002; P. A. Fraser in recorded in the previous decade, with at least litt; note that Irish records until the end of 2000 ten in eight of those years. With this in mind, it 2 © British Birds 96 • January 2003 • 2-22 Status and identification of American Wigeon seems timely to document the status and distri- Status and distribution bution of the species, particularly given the American Wigeon breeds throughout northern problems of separating genuine vagrant wild- North America, from Alaska to Hudson Bay, fowl from escapes. In addition, although male and south through the Prairies to the eastern American Wigeon in breeding plumage is a rel- seaboard (Cramp & Simmons 1977; Madge & atively easy bird to identify, the identification of Burn 1988).