Information to Users
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Bioprospecting for Hydroxynitrile Lyases by Blue Native PAGE Coupled HCN Detection
Send Orders for Reprints to [email protected] Current Biotechnology, 2015, 4, 111-117 111 Bioprospecting for Hydroxynitrile Lyases by Blue Native PAGE Coupled HCN Detection Elisa Lanfranchi1, Eva-Maria Köhler1, Barbara Darnhofer1,2,3, Kerstin Steiner1, Ruth Birner-Gruenberger1,2,3, Anton Glieder1,4 and Margit Winkler*,1 1ACIB GmbH, Graz, Austria; 2Institute for Pathology, Medical University of Graz, Graz, Austria; 3Omics Center Graz, BioTechMed, Graz, Austria; 4Institute of Molecular Biotechnology, Graz University of Technology, NAWI Graz, Graz, Austria Abstract: Hydroxynitrile lyase enzymes (HNLs) catalyze the stereoselective addition of HCN to carbonyl compounds to give valuable chiral hydroxynitriles. The discovery of new sources of HNL activity has been reported several times as the result of extensive screening of diverse plants for cyanogenic activity. Herein we report a two step-method that allows estimation of not only the native size of the active HNL enzyme but also its substrate specificity. Specifically, crude protein extracts from plant tissue are first subjected to blue native-PAGE. The resulting gel is then directly used for an activity assay in which the formation of hydrocyanic acid (HCN) is detected upon the cyanogenesis reaction of any cyanohydrin catalyzed by the enzyme of interest. The same gel may be used with different substrates, thus exploring the enzyme’s substrate scope already on the screening level. In combination with mass spectrometry, sequence information can be retrieved, which is demonstrated -

The HOTHEAD Protein: Assessing Enzymatic Activity Using Computational and Recombinant Protein Expression Approaches
The HOTHEAD Protein: Assessing Enzymatic Activity using Computational and Recombinant Protein Expression Approaches By Eric Le Dreff-Kerwin A thesis Presented to the University of Waterloo In fulfillment of the Thesis requirement for the degree of Master of Science In Biology Waterloo, Ontario, Canada, 2019 © Eric Le Dreff-Kerwin 2019 Author’s Declaration I hereby declare that I am the sole author of this thesis. This is a true copy of the thesis, including any required final revisions, as accepted by my examiners. I understand that my thesis may be made electronically available to the public. ii Abstract In Arabidopsis thaliana, a number of genes regulating cuticle synthesis have been identified by virtue of organ fusion phenotype. One such gene, HOTHEAD (HTH) was among those originally identified by this phenotype but its exact role in cuticle formation has proven challenging to determine. Previous bioinformatic work has identified that the HTH protein is a member of the GMC oxidoreductase family, and shares peptide sequence identity with a mandelonitrile lyase and an alcohol dehydrogenase that are within the same protein family. This thesis work investigated the potential enzymatic function of HTH by comparing a structural model to two structural analogs. The structure model of HTH, as determined in this study, shows that HTH shares certain conserved features of GMC proteins. The aim of this research also included isolating a recombinantly expressed HTH protein from Escherichia coli and initial work in Pichia pastoris. Protein isolation attempts in Escherichia coli failed to yield active HTH protein, potentially due to the lack of post-translational modifications. -

Assessment of Portable HAZMAT Sensors for First Responders
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Assessment of Portable HAZMAT Sensors for First Responders Author(s): Chad Huffman, Ph.D., Lars Ericson, Ph.D. Document No.: 246708 Date Received: May 2014 Award Number: 2010-IJ-CX-K024 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant report available electronically. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Assessment of Portable HAZMAT Sensors for First Responders DOJ Office of Justice Programs National Institute of Justice Sensor, Surveillance, and Biometric Technologies (SSBT) Center of Excellence (CoE) March 1, 2012 Submitted by ManTech Advanced Systems International 1000 Technology Drive, Suite 3310 Fairmont, West Virginia 26554 Telephone: (304) 368-4120 Fax: (304) 366-8096 Dr. Chad Huffman, Senior Scientist Dr. Lars Ericson, Director UNCLASSIFIED This project was supported by Award No. 2010-IJ-CX-K024, awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect those of the Department of Justice. This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. -

The List of Extremely Hazardous Substances)
APPENDIX A (THE LIST OF EXTREMELY HAZARDOUS SUBSTANCES) THRESHOLD REPORTABLE INVENTORY RELEASE QUANTITY QUANTITY CAS NUMBER CHEMICAL NAME (POUNDS) (POUNDS) 75-86-5 ACETONE CYANOHYDRIN 500 10 1752-30-3 ACETONE THIOSEMICARBAZIDE 500/500 1,000 107-02-8 ACROLEIN 500 1 79-06-1 ACRYLAMIDE 500/500 5,000 107-13-1 ACRYLONITRILE 500 100 814-68-6 ACRYLYL CHLORIDE 100 100 111-69-3 ADIPONITRILE 500 1,000 116-06-3 ALDICARB 100/500 1 309-00-2 ALDRIN 500/500 1 107-18-6 ALLYL ALCOHOL 500 100 107-11-9 ALLYLAMINE 500 500 20859-73-8 ALUMINUM PHOSPHIDE 500 100 54-62-6 AMINOPTERIN 500/500 500 78-53-5 AMITON 500 500 3734-97-2 AMITON OXALATE 100/500 100 7664-41-7 AMMONIA 500 100 300-62-9 AMPHETAMINE 500 1,000 62-53-3 ANILINE 500 5,000 88-05-1 ANILINE,2,4,6-TRIMETHYL- 500 500 7783-70-2 ANTIMONY PENTAFLUORIDE 500 500 1397-94-0 ANTIMYCIN A 500/500 1,000 86-88-4 ANTU 500/500 100 1303-28-2 ARSENIC PENTOXIDE 100/500 1 THRESHOLD REPORTABLE INVENTORY RELEASE QUANTITY QUANTITY CAS NUMBER CHEMICAL NAME (POUNDS) (POUNDS) 1327-53-3 ARSENOUS OXIDE 100/500 1 7784-34-1 ARSENOUS TRICHLORIDE 500 1 7784-42-1 ARSINE 100 100 2642-71-9 AZINPHOS-ETHYL 100/500 100 86-50-0 AZINPHOS-METHYL 10/500 1 98-87-3 BENZAL CHLORIDE 500 5,000 98-16-8 BENZENAMINE, 3-(TRIFLUOROMETHYL)- 500 500 100-14-1 BENZENE, 1-(CHLOROMETHYL)-4-NITRO- 500/500 500 98-05-5 BENZENEARSONIC ACID 10/500 10 3615-21-2 BENZIMIDAZOLE, 4,5-DICHLORO-2-(TRI- 500/500 500 FLUOROMETHYL)- 98-07-7 BENZOTRICHLORIDE 100 10 100-44-7 BENZYL CHLORIDE 500 100 140-29-4 BENZYL CYANIDE 500 500 15271-41-7 BICYCLO[2.2.1]HEPTANE-2-CARBONITRILE,5- -

Cyanohydrin - Wikipedia, the Free Encyclopedia
Cyanohydrin - Wikipedia, the free encyclopedia http://en.wikipedia.org/wiki/Cyanohydrin Cyanohydrin From Wikipedia, the free encyclopedia A cyanohydrin is a functional group found in organic compounds. Cyanohydrins have the formula R2C(OH)CN, where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids. Cyanohydrins can be formed by the cyanohydrin reaction, which involves treating a ketone or an aldehyde with hydrogen cyanide (HCN) in the presence of excess amounts of sodium cyanide (NaCN) as a catalyst: The structure of a general RR’C=O + HCN → RR’C(OH)CN cyanohydrin. In this reaction, the nucleophilic CN− ion attacks the electrophilic carbonyl carbon in the ketone, followed by protonation by HCN, thereby regenerating the cyanide anion. Cyanohydrins are also prepared by displacement of sulfite by cyanide salts:[1] Cyanohydrins are intermediates in the Strecker amino acid synthesis. Contents 1 Acetone cyanohydrins 2 Other cyanohydrins 3 References 4 External links Acetone cyanohydrins Acetone cyanohydrin, (CH3)2C(OH)CN is the cyanohydrin of acetone. It is generated as an intermediate in the industrial production of methyl methacrylate.[2] In the laboratory, this liquid serves as a source of HCN, which is inconveniently volatile.[3] Thus, acetone cyanohydrin can be used for the preparation other cyanohydrins, for of HCN to Michael acceptors, and for the formylation of arenes. Treatment of this cyanohydrin with lithium hydride affords anhydrous lithium cyanide: 1 of 3 6/3/10 6:07 PM Cyanohydrin - Wikipedia, the free encyclopedia http://en.wikipedia.org/wiki/Cyanohydrin Other cyanohydrins Mandelonitrile, with the formula C6H5CH(OH)CN, occurs in small amounts in the pits of some fruits.[1] Related cyanogenic glycosides are known, such as amygdalin. -

Process for Producing Methyl Methacrylate Verfahren Zur Herstellung Von Methylmethacrylat Procede Pour La Fabrication De Methacrylate De Methyle
~™ II 1 1 III II II 1 1 II II I Ml II I II I II (19) J European Patent Office Office europeen des brevets (11) EP 0 406 676 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publicationation and mention (51 ) |nt. CI.6: C07C 69/54, C07C 67/20 of the grant of the patent: 27.03.1996 Bulletin 1996/13 (21) Application number: 90112194.7 (22) Date of filing: 27.06.1990 (54) Process for producing methyl methacrylate Verfahren zur Herstellung von Methylmethacrylat Procede pour la fabrication de methacrylate de methyle (84) Designated Contracting States: • Ebata, Shuji, DE ES FR GB IT NL C/o Mitsubishi Gas Chem. Com. Tayuhama, Niigata-shi, Niigata-ken (JP) (30) Priority: 04.07.1989 JP 171190/89 (74) Representative: Turk, Gille, Hrabal, Leifert (43) Date of publication of application: Brucknerstrasse 20 09.01.1991 Bulletin 1991/02 D-40593 Dusseldorf (DE) (73) Proprietor: MITSUBISHI GAS CHEMICAL (56) References cited: COMPANY, INC. DE-A- 3 436 608 Chiyoda-ku, Tokyo (JP) • PATENT ABSTRACTS OF JAPAN vol. 14, no. 68 (72) Inventors: (C- 686)(401 1 ), 8 February 1 990; & JP-A-1 290653 • Higuchi, Hirofumi, (MITSUBISHI GAS CHEM) 22.11. 1989 C/o Mitsubishi Gas Chem. Com. Tayuhama, Niigata-shi, Niigata-ken (JP) Remarks: • Kida, Koichi, The file contains technical information submitted C/o Mitsubishi Gas Chem. Com. after the application was filed and not included in this Tayuhama, Niigata-shi, Niigata-ken (JP) specification CO CO CO o Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

Provisional Peer-Reviewed Toxicity Values for Acetone Cyanohydrin (Casrn 75-86-5)
EPA/690/R-12/001F l Final 10-01-2012 Provisional Peer-Reviewed Toxicity Values for Acetone Cyanohydrin (CASRN 75-86-5) Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 AUTHORS, CONTRIBUTORS, AND REVIEWERS CHEMICAL MANAGER Scott C. Wesselkamper, PhD National Center for Environmental Assessment, Cincinnati, OH DRAFT DOCUMENT PREPARED BY ICF International 9300 Lee Highway Fairfax, VA 22031 PRIMARY INTERNAL REVIEWERS Zheng (Jenny) Li, PhD, DABT National Center for Environmental Assessment, Washington, DC Anuradha Mudipalli, MSc, PhD National Center for Environmental Assessment, Research Triangle Park, NC This document was externally peer reviewed under contract to Eastern Research Group, Inc. 110 Hartwell Avenue Lexington, MA 02421-3136 Questions regarding the contents of this document may be directed to the U.S. EPA Office of Research and Development’s National Center for Environmental Assessment, Superfund Health Risk Technical Support Center (513-569-7300). ii Acetone cyanohydrin TABLE OF CONTENTS COMMONLY USED ABBREVIATIONS ................................................................................... iv BACKGROUND .............................................................................................................................1 DISCLAIMERS ...............................................................................................................................1 QUESTIONS REGARDING -
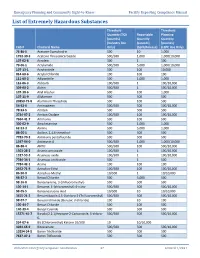
List of Extremely Hazardous Substances
Emergency Planning and Community Right-to-Know Facility Reporting Compliance Manual List of Extremely Hazardous Substances Threshold Threshold Quantity (TQ) Reportable Planning (pounds) Quantity Quantity (Industry Use (pounds) (pounds) CAS # Chemical Name Only) (Spill/Release) (LEPC Use Only) 75-86-5 Acetone Cyanohydrin 500 10 1,000 1752-30-3 Acetone Thiosemicarbazide 500/500 1,000 1,000/10,000 107-02-8 Acrolein 500 1 500 79-06-1 Acrylamide 500/500 5,000 1,000/10,000 107-13-1 Acrylonitrile 500 100 10,000 814-68-6 Acrylyl Chloride 100 100 100 111-69-3 Adiponitrile 500 1,000 1,000 116-06-3 Aldicarb 100/500 1 100/10,000 309-00-2 Aldrin 500/500 1 500/10,000 107-18-6 Allyl Alcohol 500 100 1,000 107-11-9 Allylamine 500 500 500 20859-73-8 Aluminum Phosphide 500 100 500 54-62-6 Aminopterin 500/500 500 500/10,000 78-53-5 Amiton 500 500 500 3734-97-2 Amiton Oxalate 100/500 100 100/10,000 7664-41-7 Ammonia 500 100 500 300-62-9 Amphetamine 500 1,000 1,000 62-53-3 Aniline 500 5,000 1,000 88-05-1 Aniline, 2,4,6-trimethyl- 500 500 500 7783-70-2 Antimony pentafluoride 500 500 500 1397-94-0 Antimycin A 500/500 1,000 1,000/10,000 86-88-4 ANTU 500/500 100 500/10,000 1303-28-2 Arsenic pentoxide 100/500 1 100/10,000 1327-53-3 Arsenous oxide 100/500 1 100/10,000 7784-34-1 Arsenous trichloride 500 1 500 7784-42-1 Arsine 100 100 100 2642-71-9 Azinphos-Ethyl 100/500 100 100/10,000 86-50-0 Azinphos-Methyl 10/500 1 10/10,000 98-87-3 Benzal Chloride 500 5,000 500 98-16-8 Benzenamine, 3-(trifluoromethyl)- 500 500 500 100-14-1 Benzene, 1-(chloromethyl)-4-nitro- 500/500 -

An Investigation of Transition Metal Catalysts For
AN INVESTIGATION OF TRANSITION METAL CATALYSTS FOR CYANOHYDRIN HYDRATION: THE INTERFACE OF HOMOGENEOUS AND HETEROGENOUS CATALYSIS by EMMA LEIGH DOWNS A DISSERTATION Presented to the Department of Chemistry and Biochemistry and the Graduate School of the University of Oregon in partial fulfillment of the requirements for the degree of Doctor of Philosophy June 2014 DISSERTATION APPROVAL PAGE Student: Emma Leigh Downs Title: An Investigation of Transition Metal Catalysts for Cyanohydrin Hydration: The Interface of Homogeneous and Heterogeneous Catalysis This dissertation has been accepted and approved in partial fulfillment of the requirements for the Doctor of Philosophy degree in the Department of Chemistry and Biochemistry by: Dr. Victoria J. DeRose Chairperson Dr. David R. Tyler Advisor Dr. Darren W. Johnson Core Member Dr. Mark H. Reed Institutional Representative and Kimberly Andrews Espy Vice President for Research and Innovation; Dean of the Graduate School Original approval signatures are on file with the University of Oregon Graduate School. Degree awarded June 2014 ii © 2104 Emma Leigh Downs iii DISSERTATION ABSTRACT Emma Leigh Downs Doctor of Philosophy Department of Chemistry and Biochemistry June 2014 Title: An Investigation of Transition Metal Catalysts for Cyanohydrin Hydration: The Interface of Homogeneous and Heterogeneous Catalysis Acrylic monomers are important materials that represent a large portion of the economy. The current industrial synthesis hydrates cyanohydrins with sulfuric acid, a process which results in large amounts of waste and significant energy costs. A transition metal catalyzed, acid free hydration of cyanohydrins would be beneficial from both economic and environmental standpoints. However, this reaction is challenging, as many catalysts are poisoned by the cyanide released when cyanohydrins degrade. -

Crystallization and Preliminary X-Ray Diffraction Studies of Mandelonitrile Lyase from Almonds
PROTEINS: Structure, Function, and Genetics 19:343-347 (1994) Crystallization and Preliminary X-Ray Diffraction Studies of Mandelonitrile Lyase From Almonds Hanspeter Lauble,' Kirsten Muller,' Hermann Schindelin? Siegfried Forster,' and Franz Effenberger' 'Institut fur Organische Chemie und Isotopenforschung, Uniuersitat Stuttgart, 0-70569 Stuttgart, Germany and 'Institut fur Kristallographie, Freie Universitat Berlin, 0-14195 Berlin, Germany ABSTRACT Single crystals of three differ- alyzing the dissociation of mandelonitrile into ben- ent isoenzymes of (R)-( +) mandelonitrile lyase zaldehyde and HCN.1° The protein is a single poly- (hydroxynitrile lyase) from almonds (Prunus peptide (M, 75,000) containing a FAD cofactor and is amygdalus) have been obtained by hanging heavily N-glycosylated with oligomannose units (H. drop vapor diffusion using polyethylene glycol Wajand, unpublished results). The amino-acid se- 4000 and isopropanol as co-precipitants. The quence of 12 tryptic peptides derived from MNL-A crystals belong to the monoclinic space group comprises 177 residues of the entire sequence (H. P2, with unit cell parameters a = 69.9, b = 95.1, Wajand, unpublished report). Recently the primary c = 95.6 A, and p = 118.5'. A complete set of structure of the black cherry (Prunus serotina) MNL diffraction data has been collected to 2.6 A res- has been deduced from its cDNA. l2 The sequence of olution on native crystals of isoenzyme 111. the tryptic peptides of MNL-A has been aligned with 0 1994 Wiley-Liss, Inc. respect to the black cherry -

Chandran Et Al. Supporting Info.Pdf
Supporting Information (SI) Appendix Part 1. Impact of tissue preparation, LMD, and RNA amplification on array output. p. 2 Text S1: Detailed Experimental Design and Methods Figure S1A: Correlation analysis indicates tissue preparation has minimal impact on ATH1 array output. Figure S1B: Correlation analysis indicates RNA degradation does not significantly impact array output. Figure S1C: Correlation analysis indicates two-round amplification does not significantly impact array output. Figure S1D: Independent biological replicates of LMD samples are highly correlated. Figure S1E: Validation of LMD array expression by qPCR. Table S1: Tissue preparation-associated genes Part 2. Analysis of LMD and parallel whole leaf array data. p. 13 Table S2A: Known PM-impacted genes enriched in LMD dataset Table S2B: Dataset of LMD and whole leaf genes with PM-altered expression Table S2C: LMD PM MapMan Results and Bins Table S2D: ics1 vs. WT LMD PM MapMan Results and Bins Table S2E: Infection site-specific changes for redox and calcium categories Table S2F: cis-acting regulatory element motif analysis Part 3. Process network construction p. 149 3A. Photosynthesis 3B. Cold/dehydration response Part 4. Powdery mildew infection of WT and myb3r4 mutants p. 173 Text S4: Detailed Experimental Design and Methods (supplement to manuscript) Figure S4A. Uninfected 4 week old WT and myb3r4 plants Figure S4B. myb3r4 mutants exhibit reduced visible PM growth and reproduction Figure S4C. PM-infected WT and myb3r4 mutants do not exhibit cell death Figure S4D. Endoreduplication occurs at site of PM infection not distal to infection Figure S4E. Ploidy correlates with nuclear size. Part 1. Impact of tissue preparation, LMD, and RNA amplification on array output.