(Trigonellafoenum-Graecum L.) in the CANADIAN PRAIRIES
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Botanical Nano Ozonated Erotic Oil Composition with Maximum Bioavailability for Men
MOJ Bioequivalence & Bioavailability Review Article Open Access A botanical nano ozonated erotic oil composition with maximum bioavailability for men Abstract Volume 5 Issue 1 - 2018 The present patent–pending invention relates to a topical pharmaceutical nano Awad Mansour,1 Ammar Mansour2 ozonated composition(with maximum bioavailability) for male sexual response, 1University of Akron, USA including male sexual dysfunction, such as male sexual arousal, vitality, erection 2Essraa Hospital, Jordan disorders, premature ejaculation, and sexual pain disorders, and enhancing male sexual pleasure and satisfaction of the male sexual experience as well as penis enlargement. Correspondence: Awad Mansour, University of Akron, OH, The composition for treating this problem preferably formed of a natural oil mix, USA, Tel 9622–7278278, Email [email protected] fenugreek oil, watermelon oil, ginger oil and ozonated olive oil. In particular the composition is applied on penis and testicles to enhance pleasure and delay ejaculation Received: November 25, 2017 | Published: February 07, 2018 for men during intercourse. Observed results showed excellent results for all ages of men with no adverse or side effects. Abbreviations: ED, erection dysfunction; PE, premature vasodilation, smooth muscle relaxation, etc. Australian researchers at the University of Queensland1 working with colleagues at Applied ejaculation; ASN, applied science and nutrition Science and Nutrition (ASN), a Brisbane–based company that Description of the invention specializes in scientific -

Atlantic Canada
Appendix I.1 ACCDC Report Beaver Dam Mine Site and Haul Road (DATA REPORT 6749: Marinette, NS)- January 4, 2021 Completed for the Updated 2021 Beaver Dam Mine EIS DATA REPORT 6749: Marinette, NS Prepared 4 January 2021 by C. Robicheau, Data Manager CONTENTS OF REPORT 1.0 Preface 1.1 Data List 1.2 Restrictions 1.3 Additional Information Map 1: Buffered Study Area 2.0 Rare and Endangered Species 2.1 Flora 2.2 Fauna Map 2: Flora and Fauna 3.0 Special Areas 3.1 Managed Areas 3.2 Significant Areas Map 3: Special Areas 4.0 Rare Species Lists 4.1 Fauna 4.2 Flora 4.3 Location Sensitive Species Map 1. A 100 km buffer around the study area 4.4 Source Bibliography 5.0 Rare Species within 100 km 5.1 Source Bibliography 1.0 PREFACE The Atlantic Canada Conservation Data Centre (AC CDC; www.accdc.com) is part of a network of NatureServe data centres and heritage programs serving 50 states in the U.S.A, 10 provinces and 1 territory in Canada, plus several Central and South American countries. The NatureServe network is more than 30 years old and shares a common conservation data methodology. The AC CDC was founded in 1997, and maintains data for the jurisdictions of New Brunswick, Nova Scotia, Prince Edward Island, and Newfoundland and Labrador. Although a non-governmental agency, the AC CDC is supported by 6 federal agencies and 4 provincial governments, as well as through outside grants and data processing fees. Upon request and for a fee, the AC CDC queries its database and produces customized reports of the rare and endangered flora and fauna known to occur in or near a specified study area. -

WO 2008/094873 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date PCT (10) International Publication Number 7 August 2008 (07.08.2008) WO 2008/094873 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every AOlN 65/00 (2006.01) A61K 36/00 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT,AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, (21) International Application Number: CH, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, PCT/US2008/052244 EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, (22) International Filing Date: 29 January 2008 (29.01.2008) LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PG, PH, (25) Filing Language: English PL, PT, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, SV, SY, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, (26) Publication Language: English ZA, ZM, ZW (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 60/887,036 29 January 2007 (29.01.2007) US GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), (71) Applicant and European (AT,BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, (72) Inventor: LIGUMS, John, E. -

Metabolite Profiling in Trigonella Seeds Via UPLC-MS and GC-MS Analyzed Using Multivariate Data Analyses
Anal Bioanal Chem (2016) 408:8065–8078 DOI 10.1007/s00216-016-9910-4 RESEARCH PAPER Metabolite profiling in Trigonella seeds via UPLC-MS and GC-MS analyzed using multivariate data analyses Mohamed A. Farag1 & Dalia M. Rasheed 2 & Matthias Kropf3 & Andreas G. Heiss4,5 Received: 2 July 2016 /Revised: 14 August 2016 /Accepted: 26 August 2016 /Published online: 10 September 2016 # Springer-Verlag Berlin Heidelberg 2016 Abstract Trigonella foenum-graecum is a plant of consider- supervised orthogonal projection to latent structures- able value for its nutritive composition as well as medicinal discriminant analysis (OPLS-DA). A distinct separation effects. This study aims to examine Trigonella seeds using a among the investigated Trigonella species was revealed, with metabolome-based ultra-performance liquid chromatography- T. foenum-graecum samples found most enriched in apigenin- mass spectrometry (UPLC-MS) in parallel to gas chromatog- C-glycosides, viz. vicenins 1/3 and 2, compared to the other raphy-mass spectrometry (GC-MS) coupled with multivariate two species. In contrast to UPLC-MS, GC-MS was less effi- data analyses. The metabolomic differences of seeds derived cient to classify specimens, with differences among specimens from three Trigonella species, i.e., T. caerulea, T. corniculata, mostly attributed to fatty acyl esters. GC-MS analysis of and T. foenum-graecum, were assessed. Under specified con- Trigonella seed extracts led to the identification of 91 metab- ditions, we were able to identify 93 metabolites including 5 olites belonging mostly to fatty acyl esters, free fatty acids peptides, 2 phenolic acids, 22 C/O-flavonoid conjugates, 26 followed by organic acids, sugars, and amino acids. -

MSRP Appendix E
Appendix E. Exotic Plant Species Reported from the South Florida Ecosystem. Community types are indicated where known Species High Pine Scrub Scrubby high pine Beach dune/ Coastal strand Maritime hammock Mesic temperate hammock Tropical hardwood Pine rocklands Scrubby flatwoods Mesic pine flatwoods Hydric pine flatwoods Dry prairie Cutthroat grass Wet prairie Freshwater marsh Seepage swamp Flowing water swamp Pond swamp Mangrove Salt marsh Abelmoschus esculentus Abrus precatorius X X X X X X X X X X X X Abutilon hirtum Abutilon theophrasti Acacia auriculiformis X X X X X X X X X Acacia retinoides Acacia sphaerocephala Acalypha alopecuroidea Acalypha amentacea ssp. wilkesiana Acanthospermum australe Acanthospermum hispidum Achyranthes aspera var. X aspera Achyranthes aspera var. pubescens Acmella pilosa Page E-1 Species High Pine Scrub Scrubby high pine Beach dune/ Coastal strand Maritime hammock Mesic temperate hammock Tropical hardwood Pine rocklands Scrubby flatwoods Mesic pine flatwoods Hydric pine flatwoods Dry prairie Cutthroat grass Wet prairie Freshwater marsh Seepage swamp Flowing water swamp Pond swamp Mangrove Salt marsh Acrocomia aculeata X Adenanthera pavonina X X Adiantum anceps X Adiantum caudatum Adiantum trapeziforme X Agave americana Agave angustifolia cv. X marginata Agave desmettiana Agave sisalana X X X X X X Agdestis clematidea X Ageratum conyzoides Ageratum houstonianum Aglaonema commutatum var. maculatum Ailanthus altissima Albizia julibrissin Albizia lebbeck X X X X X X X Albizia lebbeckoides Albizia procera Page -
Show Activity
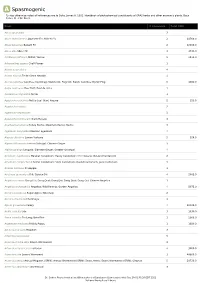
A Spasmogenic *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Abies spectabilis 3 Abies sachalinensis Japanese Fir; Shin-Yo-Yu 2 14760.0 Abies balsamea Balsam Fir 2 12000.0 Abies alba Silver-Fir 3 3736.0 Achillea millefolium Milfoil; Yarrow 5 8530.0 Achyranthes aspera Chaff Flower 1 Acinos suaveolens 4 Acinos alpinus Te de Sierra Nevada 4 Acorus calamus Calamus; Sweetflag; Sweetroot; Flagroot; Sweet Calamus; Myrtle Flag 5 1980.0 Aegle marmelos Bael fruit; Bael de India 1 Aeolanthus myriantha Ninde 1 Agastache urticifolia Nettle-Leaf Giant Hyssop 2 156.0 Agastache rugosa 2 Agastache nepetoides 3 Agastache foeniculum Giant Hyssop 3 Agathosma betulina Honey Buchu; Mountain Buchu; Buchu 3 Ageratum conyzoides Mexican ageratum 4 Aloysia citrodora Lemon Verbena 5 924.0 Alpinia officinarum Lesser Galangal; Chinese Ginger 3 Alpinia galanga Languas; Siamese Ginger; Greater Galangal 4 Amomum xanthioides Malabar Cardamom; Tavoy Cardamom; Chin Kousha; Bastard Cardamom 2 Amomum compactum Chester Cardamom; Siam Cardamom; Round Cardamom; Java Cardamom 3 Ananas comosus Pineapple 1 Anethum graveolens Dill; Garden Dill 4 2002.0 Angelica sinensis Dang Gui; Dong Quai; Dang Qui; Dang Quai; Dong Gui; Chinese Angelica 1 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 4 8073.0 Annona squamosa Sugar-Apple; Sweetsop 2 Annona cherimola Cherimoya 1 Apium graveolens Celery 4 30100.0 Aralia cordata Udo 3 1030.0 Areca catechu Pin-Lang; Betel Nut 1 2000.0 Argemone mexicana Prickly Poppy 1 1680.0 Artemisia vulgaris Mugwort 3 Artemisia salsoloides 5 Artemisia herba-alba Desert Wormwood 3 Artemisia dracunculus Tarragon 4 3000.0 Artemisia cina Levant Wormseed 1 48000.0 Artemisia annua Annual Mugwort (GRIN); Annual Wormwood (GRIN); Sweet Annie; Sweet Wormwood (GRIN); Qinghao 5 20720.0 Artemisia absinthium Wormwood 3 Dr. -

Newsletter of the Biological Survey of Canada
Newsletter of the Biological Survey of Canada Vol. 40(1) Summer 2021 The Newsletter of the BSC is published twice a year by the In this issue Biological Survey of Canada, an incorporated not-for-profit From the editor’s desk............2 group devoted to promoting biodiversity science in Canada. Membership..........................3 President’s report...................4 BSC Facebook & Twitter...........5 Reminder: 2021 AGM Contributing to the BSC The Annual General Meeting will be held on June 23, 2021 Newsletter............................5 Reminder: 2021 AGM..............6 Request for specimens: ........6 Feature Articles: Student Corner 1. City Nature Challenge Bioblitz Shawn Abraham: New Student 2021-The view from 53.5 °N, Liaison for the BSC..........................7 by Greg Pohl......................14 Mayflies (mainlyHexagenia sp., Ephemeroptera: Ephemeridae): an 2. Arthropod Survey at Fort Ellice, MB important food source for adult by Robert E. Wrigley & colleagues walleye in NW Ontario lakes, by A. ................................................18 Ricker-Held & D.Beresford................8 Project Updates New book on Staphylinids published Student Corner by J. Klimaszewski & colleagues......11 New Student Liaison: Assessment of Chironomidae (Dip- Shawn Abraham .............................7 tera) of Far Northern Ontario by A. Namayandeh & D. Beresford.......11 Mayflies (mainlyHexagenia sp., Ephemerop- New Project tera: Ephemeridae): an important food source Help GloWorm document the distribu- for adult walleye in NW Ontario lakes, tion & status of native earthworms in by A. Ricker-Held & D.Beresford................8 Canada, by H.Proctor & colleagues...12 Feature Articles 1. City Nature Challenge Bioblitz Tales from the Field: Take me to the River, by Todd Lawton ............................26 2021-The view from 53.5 °N, by Greg Pohl..............................14 2. -

Effects of Fenugreek Seed on the Severity and Systemic Symptoms of Dysmenorrhea
Original Article Effects of Fenugreek Seed on the Severity and Systemic Symptoms of Dysmenorrhea Sima Younesy 1, Sedigheh Amiraliakbari 1*, Somayeh Esmaeili 2,3, Hamid Alavimajd 4, Soheila Nouraei 1 1- Department of Midwifery, School of Nursing and Midwifery, Shahid Beheshti University of Medical Sciences, Tehran, Iran 2- Department of Traditional Pharmacy, School of Traditional Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran 3- Traditional Medicine and Materia Medica Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran 4- Department of Biostatistics, Faculty of Paramedicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran Abstract Background: Primary dysmenorrhea is a prevalent disorder and its unfavorable ef- fects deteriorates the quality of life in many people across the world. Based on some evidence on the characteristics of fenugreek as a medical plant with anti-inflammato- ry and analgesic properties, this double-blind, randomized, placebo controlled trial was conducted. The main purpose of the study was to evaluate the effects of fenu- greek seeds on the severity of primary dysmenorrhea among students. Methods: Unmarried Students were randomly assigned to two groups who received fenugreek (n=51) or placebo (n=50). For the first 3 days of menstruation, 2−3 cap- sules containing fenugreek seed powder (900 mg) were given to the subjects three times daily for two consecutive menstrual cycles. Pain severity was evaluated using a visual analog scale and systemic symptoms were assessed using a multidimension- al verbal scale. * Corresponding Author: Sedigheh Amiraliakbari, Results: Pain severity at baseline did not differ significantly between the two Department of Midwifery, groups. Pain severity was significantly reduced in both groups after the intervention; School of Nursing and however, the fenugreek group experienced significantly larger pain reduction Midwifery, Shahid (p<0.001). -

FAQ Fenugreek Seed for Increasing Milk Supply
FAQ-FENUGREEK INFORMATION Fenugreek Seed for Increasing Milk Supply By Kelly Bonyata, IBCLC EFFECT ON MILK PRODUCTION Fenugreek (Trigonella foenum-graecum L.) appears to be the herb that is most often used to increase milk supply. It has been reported to be an excellent galactagogue for some mothers, and has been used as such for centuries. The few studies that have been done have had mixed results [Swafford 2000, Reeder -pharmaceutical methods of increasing milk supply should be tried first, as there can be significant side effects from both herbal remedie2011, Turkyılmazs and prescription 2011] . Keep medications in mind thatused in to almost increase all milkcases, supply. non See the Academy of Breastfeeding Medicine’s protocol #9 on the use of galactogogues. Mothers generally notice an increase in production 24-72 hours after starting the herb, but it can take two weeks for others to see a change. Some mothers do not see a change in milk production when taking fenugreek. Dosages of less than 3500 mg per DAY have been reported to produce no effect in many women. One way reported to determine if you’re taking the correct dosage is to slowly increase the amount of fenugreek until your sweat and urine begin to smell like maple syrup. If you’re having problems with any side effects, discontinue use and consider alternative methods of increasing milk supply. Fenugreek has been used either short-term to boost milk supply or long-term to augment supply and/or pumping yields. There are no studies indicating problems with long-term usage. -

Idaho PM Technical Note 2B (Revise): Plants for Pollinators in the Inland Northwest
TECHNICAL NOTE USDA – Natural Resources Conservation Service Boise, Idaho - Spokane, Washington ______________________________________________________________________________ TN PLANT MATERIALS NO. 2B OCTOBER 2011 REVISION Plants for Pollinators in the Inland Northwest Dan Ogle, Plant Materials Specialist, NRCS, Boise, Idaho Pamela Pavek, Agronomist, NRCS Plant Materials Center, Pullman, Washington Richard Fleenor, Plant Materials Specialist, NRCS, Spokane, Washington Mark Stannard, Manager, NRCS Plant Materials Center, Pullman, Washington Tim Dring, State Biologist, NRCS, Spokane, Washington Jim Cane, Bee Biology and Systematics Lab, ARS, Logan, Utah Karen Fullen, State Biologist, NRCS, Boise, Idaho Loren St. John, Manager, NRCS Plant Materials Center, Aberdeen, Idaho Derek Tilley, Agronomist, NRCS Plant Materials Center, Aberdeen, Idaho Brownbelted bumble bee (Bombus griseocollis) visiting a blanketflower (Gaillardia aristata). Pamela Pavek The purpose of this Technical Note is to provide guidance for the design and implementation of conservation plantings to enhance habitat for pollinators including: bees, wasps, butterflies, moths and hummingbirds. Plant species included in this document are adapted to the Inland Northwest, which encompasses northern Idaho, northeastern Oregon and eastern Washington. For species adapted to southern Idaho, southeastern Oregon, northern Nevada and northern Utah, refer to Idaho Plant Materials Technical Note 2A. For lists of species adapted to western Washington and western Oregon, refer to the Oregon -

Spice Large.Pdf
Gernot Katzer’s Spice List (http://gernot-katzers-spice-pages.com/engl/) 1/70 (November 2015) Important notice Copyright issues This document is a byproduct of my WWW spice pages. It lists names of spices in about 100 different languages as well as the sci- This document, whether printed or in machine-readable form, may entific names used by botanists and pharmacists, and gives for each be copied and distributed without charge, provided the above no- local name the language where it is taken from and the botanical tice and my address are retained. If the file content (not the layout) name. This index does not tell you whether the plant in question is is modified, this should be indicated in the header. discussed extensively or is just treated as a side-note in the context of another spice article. Employees of Microsoft Corporation are excluded from the Another point to make perfectly clear is that although I give my above paragraph. On all employees of Microsoft Corporation, a best to present only reliable information here, I can take no warrant licence charge of US$ 50 per copy for copying or distributing this of any kind that this file, or the list as printed, or my whole WEB file in all possible forms is levied. Failure to pay this licence charge pages or anything else of my spice collection are correct, harm- is liable to juristical prosecution; please contact me personally for less, acceptable for non-adults or suitable for any specific purpose. details and mode of paying. All other usage restrictions and dis- Remember: Anything free comes without guarantee! claimers decribed here apply unchanged. -

Effect of Mint and Fenugreek and Mixture on Production and Immunity of Broilers
Journal of Kerbala University , Vol. 12 No.2 Scientific . 2014 EFFECT OF MINT AND FENUGREEK AND MIXTURE ON PRODUCTION AND IMMUNITY OF BROILERS تأثير النعناع والحلبة وخليطهما على اﻷداء اﻹنتاجي والمناعي لفروج اللحم Assist.Lec. Ali .R .Abed/Veterinary Medicine College- Kerbala University Assist.Lec. Fateh .O .Kadhim / Veterinary Medicine College- Kerbala University Abstract : This study conducted to determine the effect of dietary mint, fenugreek and combination on productive and immune parameters of broiler chicks (Ross308).A total of 80 broiler chicks of one day old have been reared for 35 day under good hygienic condition .The chicks were randomly divided into four groups :Treatment 1 (Mint 1%) ,Treatment 2 (fenugreek 1% ) ,Treatment 3 (Mint 1%+ fenugreek 1%) & Treatment 4(Control), each of one have 2o chicks with 2 replicate for each treatment .the addition of dry leaves of Mint and fenugreek started at one day until end of experiment, while the control group given the standard feed only .the productive parameters are measured weekly, and immune status of chicks are measured at 21 and 35 day by ELISA technique. The results have appeared that there is an improvement in performance and immunity traits for all treated groups if compared with the control group. However, the chicks feed with 1% mint and 1% fenugreek performed better than those fed with others concerning live body weight and body weight gain. with regards to feed consumption, the first treatment that supplemented with 1% mint achieved better one. the second treatment that supplemented with 1% fenugreek recorded high antibody titter against Newcastle disease virus and Gumboro disease virus at 21 and 35 day of broilers age .