In Vitro Propagation of the Rare and Endangered Grevillea Scapigera
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Propagation of Zingiberaceae and Heliconiaceae1
14 Sociedade Brasileira de Floricultura e Plantas Ornamentais Propagation of Zingiberaceae and Heliconiaceae1 RICHARD A.CRILEY Department of Horticulture, University of Hawaii, Honolulu, Hawaii USA 96822 Increased interest in tropical cut vegetative growths will produce plants flower export in developing nations has identical to the parent. A few lesser gen- increased the demand for clean planting era bear aerial bulbil-like structures in stock. The most popular items have been bract axils. various gingers (Alpinia, Curcuma, and Heliconia) . This paper reviews seed and Seed vegetative methods of propagation for Self-incompatibility has been re- each group. Auxins such as IBA and ported in Costus (WOOD, 1992), Alpinia NAA enhanced root development on aerial purpurata (HIRANO, 1991), and Zingiber offshoots of Alpinia at the rate 500 ppm zerumbet (IKEDA & T ANA BE, 1989); while while the cytokinin, PBA, enhanced ba- other gingers set seed readily. sal shoot development at 100 ppm. Rhi- zomes of Heliconia survived treatment in The seeds of Alpinia, Etlingera, and Hedychium are borne in round or elon- 4811 C hot water for periods up to 1 hour gated capsules which split when the seeds and 5011 C up to 30 minutes in an experi- are ripe and ready for dispersai. ln some ment to determine their tolerance to tem- species a fleshy aril, bright orange or scar- pera tures for eradicating nematodes. Iet in color, covers .the seed, perhaps to Pseudostems soaks in 400 mg/LN-6- make it more attractive to birds. The seeds benzylaminopurine improved basal bud of gingers are black, about 3 mm in length break on heliconia rhizomes. -

Clemson University Plant Problem Clinic, Nematode Assay Lab and Molecular Plant Pathogen Detection Lab Semi-Annual Report for 2013 (January – June)
Clemson University Plant Problem Clinic, Nematode Assay Lab and Molecular Plant Pathogen Detection Lab Semi-Annual Report For 2013 (January – June) 1 Part 1: General Information Information 3 Diagnostic Input 4 Consultant Input 4 Monthly Sample Numbers 2013 5 Monthly Sample Numbers since 2007 6 Yearly Sample Numbers since 2007 7 Nematode Monthly Sample Numbers 2013 8 Nematode Yearly Sample Numbers since 2003 9 MPPD Monthly Sample Numbers 2013 10 MPPD Yearly Sample Numbers since 2010 11 Client Types 12 Submitter Types 13 Diagnoses/Identifications Requested 14 Sample Categories 15 Sample State Origin 16 Methods Used 17 Part 2: Diagnoses and Identifications Ornamentals and Trees 18 Turf 30 Vegetables and Herbs 34 Fruits and Nuts 36 Field Crops, Pastures and Forage 38 Plant and Mushroom Identifications 39 Insect Identifications 40 Regulatory Concern 42 2 Clemson University Plant Problem Clinic, Nematode Assay Lab and Molecular Plant Pathogen Detection Lab Semi-Annual Report For 2013 (January-June) The Plant Problem Clinic serves the people of South Carolina as a multidisciplinary lab that provides diagnoses of plant diseases and identifications of weeds and insect pests of plants and structures. Plant pathogens, insect pests and weeds can significantly reduce plant growth and development. Household insects can infest our food and cause structural damage to our homes. The Plant Problem Clinic addresses these problems by providing identifications, followed by management recommendations. The Clinic also serves as an information resource for Clemson University Extension, teaching, regulatory and research personnel. As a part of the Department of Plant Industry in Regulatory Services, the Plant Problem Clinic also helps to detect and document new plant pests and diseases in South Carolina. -

Ne Wsletter No . 92
AssociationAustralian of NativeSocieties Plants for Growing Society (Australia)Australian IncPlants Ref No. ISSN 0725-8755 Newsletter No. 92 – August 2012 GSG Vic Programme 2012 GSG SE Qld Programme 2012 Leader: Neil Marriott Morning tea at 9.30am, meetings commence at 693 Panrock Reservoir Rd, Stawell, Vic. 3380 10.00am. For more information contact Bryson Phone: 03 5356 2404 or 0458 177 989 Easton on (07) 3121 4480 or 0402242180. Email: [email protected] Sunday, 26 August Contact Neil for queries about program for the year. This meeting has been cancelled as many members Any members who would like to visit the official have another function to attend over the weekend. collection, obtain cutting material or seed, assist in its maintenance, and stay in our cottage for a few days The October 2012 meeting – has been are invited to contact Neil. After the massive rains at replaced by a joint excursion through SEQ & the end of 2010 and the start of 2011 the conditions northern NSW commencing on Wednesday, 7 are perfect for large scale replanting of the collection. November 2012. GSG members planning to attend Offers of assistance would be most welcome. are asked to contact Jan Glazebrook & Dennis Cox Newsletter No. 92 No. Newsletter on Ph (07) 5546 8590 for full details closer to this Friday, 29 September to Monday, 1 October event. See also page 3 for more details. SUBJECT: Spring Grevillea Crawl Sunday, 25 November FRI ARVO: Meet at Neil and Wendy Marriott’s Panrock VENUE: Home of Robyn Wieck Ridge, 693 Panrock Reservoir Rd, Stawell Lot 4 Ajuga Court, Brookvale Park Oakey for welcome and wander around the HONE (07) 4691 2940 gardens. -

Scope Item Implementation
NARROGIN DISTRICT THREATENED FLORA MANAGEMENT PROGRAM Annual Report 2004 Marie Strelein and Greg Durell For the Narrogin District Threatened Flora Recovery Team Department of Conservation and Land Management PO Box 100 Narrogin WA 6312 SUMMARY 2004 Threatened Flora recovery within the Narrogin District is a collaborative project between the Department of Conservation and Land Management (CALM), the Commonwealth Department of Environment and Heritage (through the NHT program) the Avon Catchment Council (ACC), the Southwest Catchment Council (SWCC), the Botanic Gardens and Parks Authority (BGPA) and the community. CALM supports the program by providing both direct and indirect funding, including the full time employment of a Conservation Officer. Funds have been received from the Avon and Southwest Catchment Councils through NHT 2 and allocated to on-ground recovery actions. Funding has also been obtained from NHT for the development of Interim Recovery plans for several Narrogin District threatened plant species. The BGPA has provided direct costs to the program for two species recovery projects. The community has provided significant in- kind volunteer support to implement many of the recovery actions. CALM’s Narrogin District manages ten Critically Endangered flora (CR), eleven Endangered flora (EN) and sixteen Vulnerable Flora (VU) flora. All are Declared as Rare Flora under the Wildlife Conservation Act (1950). In addition, 213 flora species are listed for the Narrogin District on CALM’s Priority Flora List. Many of these require additional monitoring and survey to determine their threatened status. Highlights of the program for 2004 are: • A report summarising the Darwinia carnea (CR) translocation process from 1997 through to 2004 and assessing whether the translocation has met the aims outlined in the translocation proposal was completed in December 2004 by Leonie Monks. -

Foliar and Shoot Allometry of Pollarded Black Locust, Robinia Pseudoacacia L
Agroforestry Systems (2006) 68:37–42 Ó Springer 2006 DOI 10.1007/s10457-006-0001-y -1 Foliar and shoot allometry of pollarded black locust, Robinia pseudoacacia L. D.M. Burner1,*, D.H. Pote1 and A. Ares2 1United States Department of Agriculture, Agricultural Research Service, Dale Bumpers Small Farms Research Center, 6883 South State Highway 23, Booneville, AR 72927, USA; 2Weyerhaeuser Company, 505 N. Pearl Street, Centralia, WA 98531, USA; *Author for correspondence (e-mail: [email protected]; phone: +1-479-675-3834; fax: +1-479-675-2940) Received 22 June 2004; accepted in revised form 17 January 2006 Key words: Allometric relationship, Foliar mass, Nonlinear analysis, Shoot diameter, Shoot mass Abstract Browse of multipurpose tree species such as black locust could be used to broaden grazing options, but the temporal distribution of foliage has not been adequately studied. Our objective was to determine effects of harvest date, P fertilization (0 and 600 kg haÀ1 yrÀ1), and pollard height (shoots clipped at 5-, 50-, and 100- cm above ground) on foliar and shoot allometry of black locust. The experiment was conducted on a naturally regenerated 2-yr-old black locust stand (15,000 trees haÀ1). Basal shoot diameter and foliar mass were measured monthly in June to October 2002 and 2003. Foliar and shoot dry mass (Y) was estimated from basal shoot diameter (D) by the function Y = aDb, with regression explaining ‡95% of variance. Allometry of foliar mass was affected by harvest date, increasing at a greater rate with D in September than in June or July, but not by P fertilization or pollard height. -

Corrigin Grevillea), Proteaceae
Threatened plant translocation case study: Grevillea scapigera (Corrigin Grevillea), Proteaceae BOB DIXON AND SIEGY KRAUSS* Kings Park Science, Department of Biodiversity, Conservation and Attractions, Western Australia; Botanic Gardens and Parks Authority *Corresponding author: [email protected] The species Translocation working group and • Prostrate, short‑lived, fire‑killed, disturbance key stakeholders opportunist, woody perennial shrub. • Botanic Gardens and Parks Authority, • Endemic to Western Australia. Western Australia. • No extant natural populations in secure sites. • Department of Biodiversity, Conservation and Attractions, Western Australia. Threatening processes • Australian Nature Conservation Agency • Habitat loss and fragmentation through clearing (Commonwealth Government). for agriculture. • Local Corrigin and Landcare community. • Weeds. • Kings Park volunteer Master Gardeners. • Salinity. Biology and ecology • Seed and fruit predation. • Grazing. Grevillea scapigera grows after winter rains, and flowers September – December. Inflorescences are produced on • Maintenance of road verges. new growth and over 1000 flowers can be observed at • Fertiliser and herbicide drift. one time on an average 1.5 m diameter plant. Flowers are protandrous (male parts mature before female parts), Deciding to translocate strongly scented and produce abundant nectar. These The Corrigin Grevillea was first collected in 1954, and remain receptive for pollination for up to 4–5 days. has been known from only 13 small, mainly degraded Flowers are pollinated by a range of insects, especially roadside populations restricted to a 50 km radius area Hymenoptera and Lepidoptera. Ants frequently visit around the Wheatbelt town of Corrigin in Western flowers to feed on nectar, but are unlikely pollinators. Australia. The Wheatbelt region has been extensively Pollen has been successfully placed in cryostorage at used for agricultural purposes and over 94% of its Kings Park with no significant decline in viability. -

Plant Problem Clinic Annual Report for 2017 Introduction to the 2017 Annual Report
Plant Problem Clinic Annual Report for 2017 Introduction to the 2017 Annual Report As of February 1, 2018, the Clemson University Plant Problem Clinic changed its name to The Plant and Pest Diagnostic Clinic (PPDC). The PPDC, the Molecular Plant Pathogen Detection Lab (MPPD) and the Commercial Turf Clinic are housed in the Department of Plant Industry, while the Nematode Assay Lab, with whom we have a contract, is in the Department of Plant and Environmental Sciences. Despite the name change, our primary mission has not changed. The Clinic serves the people of South Carolina as a multidisciplinary lab that provides diagnoses of plant diseases and identifications of weeds and insect pests of plants and structures. Solutions for these problems are provided through management recommendations. As a part of the Department of Plant Industry in Regulatory Services, the PPDC also helps to detect and document new plant diseases and pests in South Carolina and serves as an information resource for Clemson University Extension, teaching, regulatory and research personnel. We were lucky to retain the part-time assistance of Madeline Dowling who prepared many of the tables for Host/Diagnosis by Crop for this report. We also hired Martha Froelich, another graduate student in Plant Pathology, who also assists with diagnostics and other aspects of lab management. Both have been extremely helpful. In 2017, the Plant Problem Clinic received 1215 samples and 27 people from eight disciplinary areas assisted by identifying diseases, insects or plants or by providing management recommendations. Appreciation is expressed to all faculty, students and staff that contributed their time and effort, enhancing the success of the Plant and Pest Diagnostic Clinic. -

Monitoring and Prioritisation of Flora Translocations: a Survey of Opinions from Practitioners and Researchers
MONITORING AND PRIORITISATION OF FLORA TRANSLOCATIONS: A SURVEY OF OPINIONS FROM PRACTITIONERS AND RESEARCHERS N. Hancock1, R.V. Gallagher1 & R.O. Makinson2 1. Department of Biological Sciences, Macquarie University 2. Royal Botanic Gardens & Domain Trust, Sydney i This document was compiled as part of a project funded by the NSW Biodiversity Research Hub in 2013-14. It is intended to be read in conjunction with the Australian Network for Plant Conservation publication Guidelines for the translocation of threatened plants in Australia (Vallee et al. 2004). Please cite this publication as: Hancock, N., Gallagher, R.V. & Makinson, R.O. (2014). Monitoring and prioritisation of flora translocations: a survey of opinions from practitioners and researchers. Report to the Biodiversity Hub of the NSW Office of Environment & Heritage. For further correspondence contact: [email protected] ii TABLE OF CONTENTS Summary v Acknowledgements v List of Tables vi RESULTS OF SURVEY OF PRACTITIONERS AND RESEARCHERS Section 1 A synthesis of opinions on monitoring of flora translocations – results from the Flora translocation survey (2013) 1. The Flora Translocation Survey (2013) 1.1 Introduction 1 1.2 Aims and objectives of the survey 1 1.3 What is a successful translocation? 2 1.4 The importance of monitoring 2 2. Survey design 2.1 Selection of survey respondents 3 2.2 Survey format 4 2.3 Data analysis 5 3. Survey results: quantitative data 3.1 General information 5 3.2 Monitoring duration by habit groups 6 3.3 Monitoring duration by breeding system 8 3.4 Monitoring duration by planting method 10 3.5 Monitoring duration by taxonomic groups 11 3.6 Other life-cycle monitoring 3.6.1 Early survivorship 13 3.6.2 Growth 13 4. -

(Proteaceae) with Emphasis on Banksia Cuneata, a Rare and Endangered Species
Heredity 79 (1997)394—40 1 Received 24 September 1996 Genetic diversity in Banksia and Dryandra (Proteaceae) with emphasis on Banksia cuneata, a rare and endangered species TINA L. MAGUIRE & MARGARET SEDGLEY* Department of Horticulture, Viticulture and Qenology, The University of Adelaide, Waite Campus, P/ant Research Centre, PMB 1, Glen Osmond, SA 5064, Australia Randomamplified polymorphic DNA (RAPD) markers were investigated as a tool for estimat- ing genetic diversity within 33 species of Banksia and three species of Diyandra. Three primers were used on DNA from 10 seeds per species, and band data were pooled to give between 52 and 89 bands per species, most of which were polymorphic. Genetic diversity was calculated using six published metrics on three species, for which allozyme data were also available. Based on between-method consistency, three metrics were chosen for analysis of the full data set. Levels of genetic diversity in Banksia and Diyandra ranged from 0.59 to 0.90. Based on this information, a detailed study was conducted on all 10 known populations of B. cuneata, a rare and endangered species, with a restricted geographical distribution in south-western Australia. Estimates of genetic diversity ranged from 0.65 to 0.74, which is high for a rare and endan- gered species. Analysis of molecular variance (AM0vA)wasused to partition RAPD variation within and between populations. Nearly all of the variation was attributable to individuals within populations, indicating a lack of population divergence. It is suggested that the combination of bird pollination and high outcrossing rates in B. cuneata maintain genetic diversity and cohesion between the populations. -

The Following Is the Initial Vaughan's Australian Plants Retail Grafted Plant
The following is the initial Vaughan’s Australian Plants retail grafted plant list for 2019. Some of the varieties are available in small numbers. Some species will be available over the next few weeks. INCLUDING SOME BANKSIA SP. There are also plants not listed which will be added to a future list. All plants are available in 140mm pots, with some sp in 175mm. Prices quoted are for 140mm pots. We do not sell tubestock. Plants placed on hold, (max 1month holding period) must be paid for in full. Call Phillip Vaughan for any further information on 0412632767 Or via e-mail [email protected] Grafted Grevilleas $25.00ea • Grevillea Albiflora • Grevillea Alpina goldfields Pink • Grevillea Alpina goldfields Red • Grevillea Alpina Grampians • Grevillea Alpina Euroa • Grevillea Aspera • Grevillea Asparagoides • Grevillea Asparagoides X Treueriana (flaming beauty) • Grevillea Baxteri Yellow (available soon) 1 • Grevillea Baxteri Orange • Grevillea Beadleana • Grevillea Biformis cymbiformis • Grevillea Billy bonkers • Grevillea Bipinnatifida "boystown" • Grevillea Bipinnatifida "boystown" (prostrate red new growth) • Grevilllea Bipinnatifida deep burgundy fls • Grevillea Bracteosa • Grevillea Bronwenae • Grevillea Beardiana orange • Grevillea Bush Lemons • Grevillea Bulli Beauty • Grevillea Calliantha • Grevillea Candelaborides • Grevillea Candicans • Grevillea Cagiana orange • Grevillea Cagiana red • Grevillea Crowleyae • Grevillea Droopy drawers • Grevillea Didymobotrya ssp involuta • Grevillea Didymobotrya ssp didymobotrya • Grevillea -
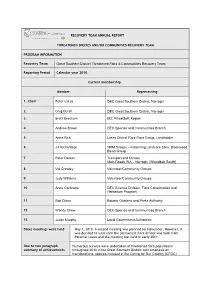
RECOVERY TEAM ANNUAL REPORT THREATENED SPECIES AND/OR COMMUNITIES RECOVERY TEAM PROGRAM INFORMATION Recovery Team Great Southern
RECOVERY TEAM ANNUAL REPORT THREATENED SPECIES AND/OR COMMUNITIES RECOVERY TEAM PROGRAM INFORMATION Recovery Team Great Southern District Threatened Flora & Communities Recovery Team Reporting Period Calendar year 2010 Current membership Member Representing 1. Chair Peter Lacey DEC Great Southern District, Narrogin 2. Greg Durell DEC Great Southern District, Narrogin 3. Brett Beecham DEC Wheatbelt Region 4 Andrew Brown DEC Species and Communities Branch 5. Anne Rick Lakes District Rare Flora Group, Landholder 6 Jill Richardson NRM Groups – Katanning Landcare Zone, Blackwood Basin Group 7 Peter Denton Transport and Utilities Main Roads WA – Narrogin (Wheatbelt South) 8 Val Crowley Volunteer/Community Groups 9 Judy Williams Volunteer/Community Groups 10 Anne Cochrane DEC Science Division, Flora Conservation and Herbarium Program 11 Bob Dixon Botanic Gardens and Parks Authority 12 Wendy Chow DEC Species and Communities Branch 13 Julian Murphy Local Government Authorities Dates meetings were held May 7, 2010. A second meeting was planned for November. However, it was decided to wait until the permanent flora officer was back from Parental Leave and the meeting was held in early 2011. One to two paragraph Numerous surveys were undertaken of threatened flora populations summary of achievements throughout 2010 in the Great Southern District, with emphasis on translocations, species included in the Caring for Our Country (CFOC) ‘Reducing the impact of rabbits on threatened flora’ project and on Critically Endangered species and other Declared Rare taxa that had not been surveyed for some time. This survey work highlighted the need for a more rigorous monitoring approach. A prioritisation process was implemented to produce a list of 66 Threatened Flora populations to be surveyed and have permanent monitoring quadrats installed. -

COMMON Plants Longleaf PINE
COMMON PlANTS OF lONGlEAF PINE - BlUE STEM RANGE Harold E. Grelen Vinson L. Duvall In preparing this handbook, the authors have received substantial assistance from predecessors and colleagues. Much of the information is from the Forest Service's "Field Book of Forage Plants on Longleaf Pine-Biuestem Ranges," by 0. Gordon Langdon, the late Miriam L. Bomhard, and John T. Cassady ( 1952). Charles Feddema, Lowell K. Halls, J. B. Hilmon, and Alfred W. Johnson, U. S. Forest Service, and Thomas N. Shiflet, U. S. Soil Conservation Service, reviewed the manu script and made important suggestions regarding content and organiza tion. Phil D. Goodrum, Bureau of Sport Fisheries and Wildlife, U. S. Fish and Wildlife Service, supplied much information on values of range plants to wildlife. Jane Roller, Forest Service, prepared illustrated keys as well as many technical descriptions and drawings. Most other drawings were by the late Leta Hughey, Forest Service, and the senior author; several are from other U.S. Department of Agriculture publica tions. U. S. FOREST SERVICE RESEARCH PAPER S0-23 COMMON PLANTS OF LONGLEAF PINE·BLUESTEM RANGE Harold E. Grelen V-inson L. Duvall SOUTHERN FO!;<.EST EXPERIMENT STAT ION Thomas, c·. Nelson, Director FOREST SERVICE U.S. DEPARTMENT OF AGRICULTURE 1966 Contents The type 1 Grasses 3 Bluestems 3 Panicums 13 Paspalums 21 Miscellaneous grasses 25 Grasslike plants 37 Forbs . 47 Legumes 47 Composites 59 Miscellaneous forbs 74 Shrubs and woody vines 78 Bibliography 90 Glossary . 91 Index of plant names 94 COMMON PLANTS OF LONGLEAF PINE· BLUEST EM RANGE This publication describes many grasses, salient taxonomic features of species mention grasslike plants, forbs, and shrubs that inhabit ed briefly as well as of those described fully.