From Aqueous Solutions: a Combined Experimental and Theoretical Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

State and Future of the Environment in the Oriental Region
Kingdom of Morocco Ministry of Energy, Mines, Ministry of Interior Water and Environment Region of Oriental Department of Environment Regional Observatory of Environment and Sustainable Development STATE AND FUTURE OF THE ENVIRONMENT IN THE ORIENTAL REGION Ministry of Energy, Mines, Water and Environment Department of Environment National Environmental Observatory of Morocco Adress : 9, Al Araar street, Sector 16, Hay Riyad, Rabat Phone : +212 (0) 5 37 57 66 41 Fax : +212 (0) 5 37 57 66 42 www.environnement.gov.ma Regional Observatory of Environment and Sustainable Development of the Oriental Region Adress : Siège du Conseil Régional, Bd, le Prince Héritier Moulay El Hassan , Oujda Phone : +212 (0) 5 36 52 48 70 SYNTHESIS REPORT FOR DECISION MAKERS Fax : +212 (0) 5 36 52 48 64 2013 Table of Contents THE ENVIRONMENTAL INTEGRATED ASSESSMENT, 06 01 A DECISION-MAKING TOOL 1.1 WHY THE NEED FOR A REGIONAL ENVIRONMENTAL INTEGRATED 06 ASSESSMENT? 1.2 A CONSULTATIVE AND PARTICIPATIVE APPROACH 06 A REGION WITH STRONG POTENTIAL, BUT WITH SIGNIFICANT 07 02 SOCIAL AND ENVIRONMENTAL ISSUES 2.1 A PREDOMINANTLY URBAN REGION 07 2.2 AN EMERGING ECONOMIC REGION 08 2.2.1 INDUSTRY 08 2.2.2 TRADING 09 2.2.3 AGRICULTURE AND LIVESTOCK 09 2.2.4 TOURISM 09 2.2.5 CRAFTMANSHIP 10 2.2.6 MINNING AND QUARRYING ACTIVITIES 10 2.2.7 SEA FISHING 11 2.2.8 TRANSPORTATION 11 03 ENVIRONMENTAL STATE AND TRENDS OF THE REGION 12 3.1 THE WORRYING FATE OF WATER RESSOURCES 12 3.1.1 QUANTITATIVE TERMS 12 3.1.2 QUALITATIVE TERMS 13 3.2 WASTEWATER SANITATION, AN ONGOING MANAGEMENT -

La Liste Des Jardins D'enfants Relevant Du Ministère De La Jeunesse Et Des Sports
la liste des Jardins d'Enfants relevant du Ministère de la Jeunesse et des Sports Délégation Etablissement Milieu Adresse Région : Rabat -Salé -Kenitra Rabat Alazhar Urbain Lotissement Alhaj Slimane n°15 C.Y.M Annahda Urbain Derb elfassi Rue joutiya n° 06 Souika Yaacoub eddar Urbain Rue 48 n° 1 Hay El Barid C.Y.M Provincial Urbain Avenue Sidi Mohamed Ben Abdellah C.Y.M Touarga Almichouar Said Touarga Urbain Almichouar Said Touarga Sale Tabrekte Urbain Hay Almazraä tabrekte Bettana Urbain Avenue Palestine Sale Achahbaa Urbain Avenue 2 Mars Rue Said Hajji Salé Alaayayda 1 Urbain Lgazzara Laayayda 1 Salé Alaayayda 2 Urbain Terrain Benaacher Alaayayda 2 Salé Skhirate Témara Almanzah Rural Commune Almanzah Sidi Yahya Rural Sidi Yahya zaër Ain Aaouda Urbain Eglise Ain Aaouda Annasr Urbain Hay Annasr Témara Hay Alfath Rural Hay Alfath skhirate Khémisset Provincial Urbain Avenue Idriss Elharti hay Essalam khémisset J.G Alaman Urbain Al hay alidari Khémisset Almahalli Urbain Rue Maarakate Badr Alkobra Khémisset Alamal Urbain Avenue la marche verte Tifelte Central « Almarkazi » Urbain N°66 rue almaazize hay alfarkh tifelte Albahraoui Rural - Arromani Rural Hay alamal Arromani Alghandour Rural Alghandour centre Alkanzara Rural Alkanzara centre Almaaziz Rural Almaaziz Alhay Alidari Ait Ikou Rural Ait Ikou centre Had Elbrachwa Rural Had Elbrachwa centre Ait Yadine Rural Ait Yadine centre Tiddasse Rural Tiddasse centre Khmisse Sidi Yahya Rural Commune Khmisse Sidi Yahya Oulmes Rural Oulmes centre Kénitra Alminaa Urbain Villa n°276 biir errami algharbiya -

The Nador West Med Port Complex Serving the Territorial Attractiveness of the Eastern Region: Qualitative Study
ISSN: 2658-8455 Volume 2, Issue 1 (January, 2021), pp. 330-343. www.ijafame.org The Nador west med port complex serving the territorial attractiveness of the eastern region: Qualitative study Tarek Lakhloufi, (PhD Student) Laboratory of International Economics and Economic Development Faculty of Legal, Economic and Social Sciences Ain-Chok Hassan II University, Casablanca, Morocco Brahim El Majidi, (PhD Student) Laboratory of Research in Territorial, Integrated and Functional Management National School of Business and Management Mohamed Premier University, Oujda, Morocco National School of Business and Management Adress : University complex BP 658 Oujda Mohamed Premier University Correspondence address: Morocco (Oujda) Téléphone : +212536506989 Fax +212536506984 [email protected] Authors are not aware of any findings that might be perceived Disclosure statement: as affecting the objectivity of this study Conflicts of interest: The author reports no conflicts of interest. Lakhloufi, T., & El Majidi, B. (2021). The Nador west med port complex serving the territorial attractiveness of the Cite this article eastern region: : Qualitative study. International Journal of Accounting, Finance, Auditing, Management and Economics, 2(1), 330-343. https://doi.org/10.5281/zenodo.4474459 DOI: 10.5281/zenodo.4474459 Published online: January 29, 2021 Copyright © 2021 – IJAFAME ISSN: 2658-8455 Volume 2, Issue 1 (January, 2021), pp. 330-343. www.ijafame.org The Nador west med port complex serving the territorial attractiveness of the eastern region: Qualitative study Abstract This article tries to assess the influence of the Nador west med (NWM) port complex on the territorial attractiveness of the eastern region in a context marked by the implementation of the Royal initiative for the development of the Eastern Region (2003), the implementation of the policy of large building sites and the territorialization of sectoral strategies. -

Construction.Pdf
Construction 2 TAILLE ET ÉVOLUTION DU MARCHÉ NOMBRE TOTAL D'ENTREPRISES (ACTIVES/ CRÉATIONS ANNUELLES INACTIVES) Année Maroc Orientale Nombre d’entreprises Maroc Orientale 2015 7 714 419 Global 119 007 7 119 2016 8 445 430 Statut actif 107 880 5 923 2017 8 196 391 Statut non actif 11 127 1 196 2018 9 585 435 2019 10 279 459 2020 6 616 336 1196 Entreprises Inactives Maroc 7714 6616 5923 Entreprises Actives Oriental 419 336 2015 2016 2017 2018 2019 2020 3 TAILLE ET ÉVOLUTION DU MARCHÉ RÉPARTITION GÉOGRAPHIQUE DES ENTREPRISES DE LA RÉGION L'ORIENTAL Province Ville Entreprises Berkane 757 Berkane Ahfir (M) 40 Berkane Aklim (M) 12 Berkane Berkane (M) 616 Berkane Boughriba 1 Berkane Chouihia 1 Berkane Fezouane 2 Berkane Laatamna 11 Berkane Madagh 18 Berkane Saidia (M) 71 Berkane Sidi Bouhria 1 Berkane Sidi Slimane Echcharraa (M) 6 Berkane Tafoughalt 1 Berkane Zegzel 5 Driouch 103 Driouch Ain Zohra 2 Driouch Azlaf 1 Driouch Ben Taieb 3 Driouch Dar El Kebdani 1 Driouch Driouch 80 Driouch Midar 8 Driouch Tafersit 1 Driouch Temsamane 6 Driouch Tsaft 1 Figuig 208 Figuig Bni Guil 3 Figuig Bni Tadjite 22 Figuig Bouanane 7 Figuig Bouarfa (M) 111 Figuig Figuig (M) 41 Figuig Talsint 22 Figuig Tendrara 3 Guercif 343 Guercif Guercif (M) 326 Guercif Houara Oulad Raho 4 Guercif Lamrija 2 Guercif Mazguitam 2 Guercif Ras Laksar 3 Guercif Saka 1 Guercif Taddart 8 Jerada 206 Jerada Ain Bni Mathar (M) 20 Jerada Gafait 4 Jerada Guenfouda 5 Jerada Jerada (M) 170 Jerada Lebkhata 1 Jerada Mrija 1 Jerada Touissit (M) 5 Nador 1 838 Nador Afsou 1 Nador Al Aaroui (M) -

Diapositiva 1
Table de matière Présentation du projet ACCMA Les scénarios d’émission du GIEC Milieu biophysique du Littoral Méditerranéen Oriental L’élévation du niveau de la mer et les événements (LMO) climatiques extrêmes Analyse et cartographie de la végétation dans les Downscaling des scénarios du GIEC au niveau de Littoral communes rurales de Beni Chiker et de Boudinar Méditerranéen Oriental Analyse et cartographie de la végétation dans le Modélisation météomarine pourtour de la lagune de Nador Analyse et cartographie de la végétation dans le Erosion côtière au niveau de la lagune de Nador et de littoral de Saidia-Ras El Ma Saidia Milieu socioéconomique du Littoral Méditerranéen Vulnérabilité des écosystèmes du LMO { l’Elévation du Oriental (LMO) Niveau de la Mer Milieu socioéconomique de la commune rurale de Beni Vulnérabilité de la végétation du littoral Saidia-Ras El Ma Chiker { l’élévation de niveau de la mer Milieu socioéconomique de la commune rurale de Vulnérabilité de la végétation de Sebkha Bouareg à Boudinar l’élévation du niveau de la mer Milieu socioéconomique des communes du pourtour Vulnérabilité socioéconomique du littoral de la lagune de de la lagune de Nador Nador et de la commune rurale de Beni Chiker à l’élévation du niveau de la mer Milieu socioéconomique du littoral Saidia-Ras El Ma Vulnérabilité socioéconomique du littoral de Saidia-Ras El Ma et de la commune rurale de Boudinar { l’élévation du niveau de la mer Aspects socio-économique de la pêche artisanale dans Vulnérabilité des communes rurales de Boudinar et de le littoral -

MPLS VPN Service
MPLS VPN Service PCCW Global’s MPLS VPN Service provides reliable and secure access to your network from anywhere in the world. This technology-independent solution enables you to handle a multitude of tasks ranging from mission-critical Enterprise Resource Planning (ERP), Customer Relationship Management (CRM), quality videoconferencing and Voice-over-IP (VoIP) to convenient email and web-based applications while addressing traditional network problems relating to speed, scalability, Quality of Service (QoS) management and traffic engineering. MPLS VPN enables routers to tag and forward incoming packets based on their class of service specification and allows you to run voice communications, video, and IT applications separately via a single connection and create faster and smoother pathways by simplifying traffic flow. Independent of other VPNs, your network enjoys a level of security equivalent to that provided by frame relay and ATM. Network diagram Database Customer Portal 24/7 online customer portal CE Router Voice Voice Regional LAN Headquarters Headquarters Data LAN Data LAN Country A LAN Country B PE CE Customer Router Service Portal PE Router Router • Router report IPSec • Traffic report Backup • QoS report PCCW Global • Application report MPLS Core Network Internet IPSec MPLS Gateway Partner Network PE Router CE Remote Router Site Access PE Router Voice CE Voice LAN Router Branch Office CE Data Branch Router Office LAN Country D Data LAN Country C Key benefits to your business n A fully-scalable solution requiring minimal investment -

Parc Industriel De Selouane
Parc Industriel de Selouane Une nouvelle génération de parcs industriels intégrés SELOUANE DRIOUCH NADOR AGROPOLE BERKANE TECHNOPOLE OUJDA Un partenariat public-privé TAOURIRT JERRADA Convention cadre : 27 juillet 2007 Convention de valorisation : 11 avril 2008 FIGUIG Investisseur Etat • SAPS composée de • Ministère de l’Industrie La stratégie MED-EST, une vision > MEDZ • Agence de l’Oriental ambitieuse pour le développement de > CCIS de Nador • Province de Nador l’Oriental • Technopole d’Oujda • Parc Industriel de Selouane • Création d’une société dédiée • Pilotage du projet • Agropole de Berkane • Aménagement • Participation au fnancement LEGENDE ZONES • Commercialisation et Gestion du hors site PME/PMI Services Positionnement : Parc généraliste pour Commerces RBs des activités industrielles propres Formation et R&D Espace vert Incubateur d’entreprises Support Services Mise en œuvre – 1ère tranche PME / PMI Aménagement Commercialisation Logistique Formation et R & D • In site : 100% • Commission d’attribution: (voirie, Assainissement, AEP, Electricité et téléphonie) • CRI, Ministère de l’Industrie, SAPS, • Programme global : 72 ha • Hors site : Agence de l’Oriental, FIRO (possibilité d’extension jusqu’ à 142 ha) > Ligne électrique / conduite AEP : 95% • Processus : Formulaires, Grille de notation, • Première tranche : 44 ha > Station de traitement /réservoir d’AEP : AO entretien avec investisseurs • Appel à projets lancé Offre de Valeur: • PME / PMI : 35 ha • Devantures et commerces : 7 ha • Services : 2 ha • Formation et R & D : 2 -

Contrat-Progarmme Regional Region De L'oriental Au
Royaume du Maroc Ministère de l’Aménagement du Territoire National, de l’Urbanisme, de l’Habitat et de la Politique de la Ville CONTRAT-PROGARMME REGIONAL REGION DE L’ORIENTAL AU TITRE DE LA PERIODE 2017 - 2021 1 Oujda, le 27 Septembre 2017 Cadre référentiel Le présent Contrat - programme s’inscrit dans le cadre : des principes consacrés par la Constitution : en matière de bonne gouvernance : Les principes fondamentaux de la bonne gouvernance des services publics sont mis en exergue dans le Titre XII intitulé «De la bonne gouvernance - Principes généraux » ; en matière de planification stratégique, d’aménagement du territoire et d’urbanisme ; en matière de reddition des comptes cités plus particulièrement dans l’article 154 de la Constitution, et qui instaure clairement la liaison entre la responsabilité et la reddition des comptes ; en matière d’accès à un logement décent, article 31, qui stipule que : « L'Etat, les établissements publics et les collectivités territoriales œuvrent à la mobilisation de tous les moyens à disposition pour faciliter l'égal accès des citoyennes et des citoyens aux conditions leur permettant de jouir des droits :... à un logement décent au développement durable, à un environnement sain, … » ; en matière de suivi et évaluation ; principes cités dans plusieurs articles de la Constitution, notamment les articles 13-101-148 et 156 ; Des orientations Royales en matière d’Aménagement du Territoire National, d’Urbanisme, de l’Habitat et de la Politique de la ville ; Des engagements du programme gouvernemental -

Opportunités D'investissement En Aquaculture Région De L'oriental
اﳌﻤﻠﻜﺔ اﳌﻐﺮﺑﻴـﺔ Royaume du Maroc Opportunités d’investissement en aquaculture Région de l’Oriental Sommaire 3 RÉGION DE L’ORIENTAL, UN POSITIONNEMENT STRATÉGIQUE 4 RÉGION DE L’ORIENTAL, UNE RÉGION EN PLEINE ESSOR 6 OFFRE AQUACOLE, DES OPPORTUNITÉS D’INVESTISSEMENT GÉNÉRATRICES DE RICHESSES ET CRÉATRICES D’EMPLOI 8 ANNEXES RÉGION DE L’ORIENTAL, UN POSITIONNEMENT STRATÉGIQUE A l’Est du Maroc, la région de l’Oriental son potentiel économique. Elle constitue s’étend sur 90.130 km², soit plus de l’interFace incontournable pour conForter le 12% du territoire national. Cette région Maroc dans sa position géostratégique de revêt une importance stratégique, autant carreFour entre l’Europe, le Grand Maghreb par sa position géographique que pour et les pays de la Méditerranée. Carte de localisation de la région de l’Oriental La région de l’Oriental se compose de la Nador, Berkane, Taourirt, Guercif, Figuig préfecture d’Oujda Angad ( chef-lieu de et Jerada. la région ), et de sept provinces: Driouch, Opportunités d’investissement en aquaculture 3 RÉGION DE L’ORIENTAL, UNE RÉGION EN PLEIN ESSOR Le positionnement géographique de Le réseau routier la région de l’Oriental avec sa façade Le réseau routier de la région de l’Oriental, Méditerranéenne et le développement de s’étendant de la côte méditerranéenne à la zone portuaire de Nador sont des atouts la frontière algérienne au sud, est bien pour l’accès aux marchés européens et développé. Egalement, la région est du pourtour méditerranéen. Par ailleurs, traversée par une autoroute de 320 km, le développement des infrastructures dans le sens Oujda-Fès. -
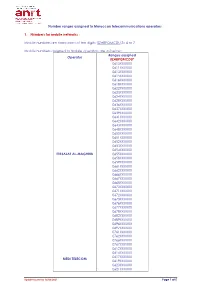
Number Ranges Assigned to Moroccan Telecommunications Operators
Number ranges assigned to Moroccan telecommunications operators 1. Numbers for mobile networks : Mobile numbers are composed of ten digits: 0ZABPQMCDU Z= 6 or 7. Mobile numbers assigned to mobile operators are as below: Ranges assigned Operator 0ZABPQMCDU* 0610XXXXXX 0611XXXXXX 0613XXXXXX 0615XXXXXX 0616XXXXXX 0618XXXXXX 0622XXXXXX 0623XXXXXX 0624XXXXXX 0628XXXXXX 0636XXXXXX 0637XXXXXX 0639XXXXXX 0641XXXXXX 0642XXXXXX 0643XXXXXX 0648XXXXXX 0650XXXXXX 0651XXXXXX 0652XXXXXX 0653XXXXXX 0654XXXXXX ITISSALAT AL-MAGHRIB 0655XXXXXX 0658XXXXXX 0659XXXXXX 0661XXXXXX 0662XXXXXX 0666XXXXXX 0667XXXXXX 0668XXXXXX 0670XXXXXX 0671XXXXXX 0672XXXXXX 0673XXXXXX 0676XXXXXX 0677XXXXXX 0678XXXXXX 0682XXXXXX 0689XXXXXX 0696XXXXXX 0697XXXXXX 0761XXXXXX 0762XXXXXX 0766XXXXXX 0767XXXXXX 0612XXXXXX 0614XXXXXX 0617XXXXXX MEDI TELECOM 0619XXXXXX 0620XXXXXX 0621XXXXXX Update issued on 14/06/2021 Page 1 of 5 0625XXXXXX 0631XXXXXX 0632XXXXXX 0644XXXXXX 0645XXXXXX 0649XXXXXX 0656XXXXXX 0657XXXXXX 0660XXXXXX 0663XXXXXX 0664XXXXXX 0665XXXXXX 0669XXXXXX 0674XXXXXX 0675XXXXXX 0679XXXXXX 0684XXXXXX 0688XXXXXX 0691XXXXXX 0693XXXXXX 0694XXXXXX 0770XXXXXX 0771XXXXXX 0772XXXXXX 0773XXXXXX 0774XXXXXX 0775XXXXXX 0777XXXXXX 0526XXXXXX 0527XXXXXX 0533XXXXXX 0534XXXXXX 0540XXXXXX 0546XXXXXX 0547XXXXXX 0550XXXXXX 0553XXXXXX 060XXXXXXX 0626XXXXXX 0627XXXXXX 0629XXXXXX 0630XXXXXX 0633XXXXXX 0634XXXXXX Wana Corporate 0635XXXXXX 0638XXXXXX 0640XXXXXX 0646XXXXXX 0647XXXXXX 0680XXXXXX 0681XXXXXX 0687XXXXXX 0690XXXXXX 0695XXXXXX 0698XXXXXX 0699XXXXXX 0700XXXXXX 0701XXXXXX 0702XXXXXX 0703XXXXXX -

Monographie 2005
MonographieMonographie ÉconomiqueÉconomique dede NadorNador AnnéeAnnée 20052005 Monographie Économique de Nador 2005 Page 2 S.M Le Roi Mohamed VI Extrait du Discours Royal prononcé à Oujda le mardi 18 mars 2003. «... Soucieux de manifester concrètement Notre haute sollicitude pour cette région qui recèle d'importantes potentialités et des ressources humaines industrieuses et fortement motivées, Nous avons décidé de lancer une Initiative Royale pour le développement de la Région de l'Oriental. S'articulant autour de quatre axes, elle vise à stimuler l'investissement et a favoriser la création de petites et moyennes entreprises par les jeunes. Elle se propose également de doter la Région des équipements de base nécessaires et d'encourager les grands projets économiques à titre prioritaire. L'initiative a pour but, en outre, de promouvoir l'éducation et la formation et de faire jouer pleinement les principes de solidarité. » « … Aussi, appelons-Nous Notre gouvernement, dans le cadre de cette initiative, à prévoir, à titre prioritaire, la réalisation, dans la Région, des infrastructures et des équipements de base nécessaires, notamment I'autoroute Fès-Oujda par Taza et la voie ferrée entre Taourirt et Nador. Il lui appartient, parallèlement de hâter la construction de la route côtière du Nord et l'élargissement et la réfection de la route reliant Nador, Oujda et Figuig. » « … Eu égard à Notre attachement au développement intégré de cette Région, Nous avons décidé la création à Nador d'une zone franche intégrant, outre le port, des espaces économiques, commerciaux et touristiques. Ce que Nous recherchons, à travers cet important projet, c’est qu’il ouvre un portail méditerranéen devant le développement de la Région et que, de surcroît, il contribue à la consolidation de l'économie nationale et au renforcement du grand complexe Tanger- Méditerranée. -

Plaquette Commerciale Zone Industrielle Selouane Nador
UN EMPLACEMENT Gibraltar Distances Temps Voie express 0 km 0 mn Tanger Oujda-Nador STRATÉGIQUE N16 Nador 10 km 24 mn Melilla Al Hoceïma Melilia 30 km 1h N16 Saïdia Nador Oujda 140 km 1h 30mn Forte de sa situation géographique stratégique N16 PARC INDUSTRIEL DE SELOUANE et distincte, la zone industrielle de Selouane est l’un des chantiers majeurs qui contribuent au A2 développement économique et social de la ville de A2 Distances Temps Nador de Nador et renforcent son attractivité. A2 Fès Parc industriel A2 Gare de Nador 10 km 27 mn De selouane Distances Temps Aéroport de Nador 12 km 24 mn Aéroport de Oujda 140 km 1h 30mn Distances Temps Port de Nador West 35 km 40 mn Med Port de Nador 15 km 23 mn Situé A proximité A proximité Main d’œuvre Des infrastructures économiques Proximité sur la voie express 3 ports à Nador, à 2 aéroports à Nador qualifiée et disponible structurantes dans la région: de l’Europe et du Maghreb Oujda-Nador Mellilia en plus du et à Oujda futur port Nador West Med, futur port Nador West station touristique Marchica, voie Med qui est en cours de express Oujda - Berkane - Nador réalisation et rocade méditerranéenne Tanger - Saïdia UN PARC UN UNE à vocation Le parc industriel de Selouane offre multisectorielle aux entreprises installées un cadre OFFRE CADRE agréable et propice à l’exercice et au COMPÉTITIVE STIMULANT développement de leurs activités. 50 Hectares Le Parc industriel de Selouane constitue l’un des chantiers phares pouvant contribuer au développement économique et social de la ville de Nador.