Ecosystem Oceanography of Seabird Hotspots: Environmental Determinants and Relationship with Antarctic Krill Within an Important Fishing Ground
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Southern Fulmar
SOUTHERN FULMAR Fulmarus glacialoides Document made by the French Southern and Antarctic Lands © TAAF Lands © Southern and Antarctic the French made by Document l l assessm iona asse a g ss b e e m lo n r • Size : 45-50 cm t F G e A n t A SOUTHERN FULMAR T • Wingspan : 114-120 cm Fulmarus glacialoides • Weight : 0.7-1kg Order : Procellariiformes — Family : Procellariidae as the ... It is also known GEOGRAPHIC RANGE : The species can be seen in the Southern Ocean but breeds on the coasts of Antarctica and outlying glaciated islands. HABITAT : Southern Fulmars nest on steep rocky slopes and cliff sides, mainly on the coast and on the Antarctic continent. They are highly nomadic outside the breeding season, generally moving north to open waters south of 30°S. © S. BLANC DIET : They eat krill, fish and squid depending on available prey. They also consume carrion and discards from Fulmar Antarctic fishing vessels. BEHAVIOR : REPRODUCTION : Most food is taken by surface-seizing whilst in The breeding season begins in November and egg- flocks. They also skim the surface in low flight laying takes place during the first two weeks of with their beak open. They also sometimes dive December. They breed in colonies on steep rocky at shallow depths to catch their prey. They are slopes and precipitous cliffs on sheltered ledges rather solitary birds that can form small groups or in hollows, sometimes with other species of outside the breeding season. Paired birds tend to petrels. Nests are a gravel-lined scrape in which © S. -

Management Plan for Antarctic Specially Protected Area No
Measure 2 (2005) Annex E Management Plan for Antarctic Specially Protected Area No. 120 POINTE-GÉOLOGIE ARCHIPELAGO, TERRE ADÉLIE Jean Rostand, Le Mauguen (former Alexis Carrel), Lamarck and Claude Bernard Islands, The Good Doctor’s Nunatak and breeding site of Emperor Penguins 1. Description of Values to be Protected In 1995, four islands, a nunatak and a breeding ground for emperor penguins were classified as an Antarctic Specially Protected Area (Measure 3 (1995), XIX ATCM, Seoul) because they were a representative example of terrestrial Antarctic ecosystems from a biological, geological and aesthetics perspective. A species of marine mammal, the Weddell seal (Leptonychotes weddelli) and various species of birds breed in the area: emperor penguin (Aptenodytes forsteri); Antarctic skua (Catharacta maccormicki); Adélie penguins (Pygoscelis adeliae); Wilson’s petrel (Oceanites oceanicus); giant petrel (Macronectes giganteus); snow petrel (Pagodrama nivea), cape petrel (Daption capense). Well-marked hills display asymmetrical transverse profiles with gently dipping northern slopes compared to the steeper southern ones. The terrain is affected by numerous cracks and fractures leading to very rough surfaces. The basement rocks consist mainly of sillimanite, cordierite and garnet-rich gneisses which are intruded by abundant dikes of pink anatexites. The lowest parts of the islands are covered by morainic boulders with a heterogenous granulometry (from a few cm to more than a m across). Long-term research and monitoring programs of birds and marine mammals have been going on for a long time already (since 1952 or 1964 according to the species). A database implemented in 1981 is directed by the Centre d'Etudes Biologiques de Chize (CEBC-CNRS). -

An Assessment for Fisheries Operating in South Georgia and South Sandwich Islands
FAO International Plan of Action-Seabirds: An assessment for fisheries operating in South Georgia and South Sandwich Islands by Nigel Varty, Ben Sullivan and Andy Black BirdLife International Global Seabird Programme Cover photo – Fishery Patrol Vessel (FPV) Pharos SG in Cumberland Bay, South Georgia This document should be cited as: Varty, N., Sullivan, B. J. and Black, A. D. (2008). FAO International Plan of Action-Seabirds: An assessment for fisheries operating in South Georgia and South Sandwich Islands. BirdLife International Global Seabird Programme. Royal Society for the Protection of Birds, The Lodge, Sandy, Bedfordshire, UK. 2 Executive Summary As a result of international concern over the cause and level of seabird mortality in longline fisheries, the United Nations Food and Agricultural Organisation (FAO) Committee of Fisheries (COFI) developed an International Plan of Action-Seabirds. The IPOA-Seabirds stipulates that countries with longline fisheries (conducted by their own or foreign vessels) or a fleet that fishes elsewhere should carry out an assessment of these fisheries to determine if a bycatch problem exists and, if so, to determine its extent and nature. If a problem is identified, countries should adopt a National Plan of Action – Seabirds for reducing the incidental catch of seabirds in their fisheries. South Georgia and the South Sandwich Islands (SGSSI) are a United Kingdom Overseas Territory and the combined area covered by the Territorial Sea and Maritime Zone of South Georgia is referred to as the South Georgia Maritime Zone (SGMZ) and fisheries within the SGMZ are managed by the Government of South Georgia and South Sandwich Islands (GSGSSI) within the framework of the Convention on the Conservation of Antarctic Marine Living (CCAMLR). -

Appendix, French Names, Supplement
685 APPENDIX Part 1. Speciesreported from the A.O.U. Check-list area with insufficient evidencefor placementon the main list. Specieson this list havebeen reported (published) as occurring in the geographicarea coveredby this Check-list.However, their occurrenceis considered hypotheticalfor one of more of the following reasons: 1. Physicalevidence for their presence(e.g., specimen,photograph, video-tape, audio- recording)is lacking,of disputedorigin, or unknown.See the Prefacefor furtherdiscussion. 2. The naturaloccurrence (unrestrained by humans)of the speciesis disputed. 3. An introducedpopulation has failed to becomeestablished. 4. Inclusionin previouseditions of the Check-listwas basedexclusively on recordsfrom Greenland, which is now outside the A.O.U. Check-list area. Phoebastria irrorata (Salvin). Waved Albatross. Diornedeairrorata Salvin, 1883, Proc. Zool. Soc. London, p. 430. (Callao Bay, Peru.) This speciesbreeds on Hood Island in the Galapagosand on Isla de la Plata off Ecuador, and rangesat seaalong the coastsof Ecuadorand Peru. A specimenwas takenjust outside the North American area at Octavia Rocks, Colombia, near the Panama-Colombiaboundary (8 March 1941, R. C. Murphy). There are sight reportsfrom Panama,west of Pitias Bay, Dari6n, 26 February1941 (Ridgely 1976), and southwestof the Pearl Islands,27 September 1964. Also known as GalapagosAlbatross. ThalassarchechrysosWma (Forster). Gray-headed Albatross. Diornedeachrysostorna J. R. Forster,1785, M6m. Math. Phys. Acad. Sci. Paris 10: 571, pl. 14. (voisinagedu cerclepolaire antarctique & dansl'Ocean Pacifique= Isla de los Estados[= StatenIsland], off Tierra del Fuego.) This speciesbreeds on islandsoff CapeHorn, in the SouthAtlantic, in the southernIndian Ocean,and off New Zealand.Reports from Oregon(mouth of the ColumbiaRiver), California (coastnear Golden Gate), and Panama(Bay of Chiriqu0 are unsatisfactory(see A.O.U. -

Order PROCELLARIIFORMES: Albatrosses
Text extracted from Gill B.J.; Bell, B.D.; Chambers, G.K.; Medway, D.G.; Palma, R.L.; Scofield, R.P.; Tennyson, A.J.D.; Worthy, T.H. 2010. Checklist of the birds of New Zealand, Norfolk and Macquarie Islands, and the Ross Dependency, Antarctica. 4th edition. Wellington, Te Papa Press and Ornithological Society of New Zealand. Pages 64, 78-79 & 82-83. Order PROCELLARIIFORMES: Albatrosses, Petrels, Prions and Shearwaters Checklist Committee (1990) recognised three families within the Procellariiformes, however, four families are recognised here, with the reinstatement of Pelecanoididae, following many other recent authorities (e.g. Marchant & Higgins 1990, del Hoyo et al. 1992, Viot et al. 1993, Warham 1996: 484, Nunn & Stanley 1998, Dickinson 2003, Brooke 2004, Onley & Scofield 2007). The relationships of the families within the Procellariiformes are debated (e.g. Sibley & Alquist 1990, Christidis & Boles 1994, Nunn & Stanley 1998, Livezey & Zusi 2001, Kennedy & Page 2002, Rheindt & Austin 2005), so a traditional arrangement (Jouanin & Mougin 1979, Marchant & Higgins 1990, Warham 1990, del Hoyo et al. 1992, Warham 1996: 505, Dickinson 2003, Brooke 2004) has been adopted. The taxonomic recommendations (based on molecular analysis) on the Procellariiformes of Penhallurick & Wink (2004) have been heavily criticised (Rheindt & Austin 2005) and have seldom been followed here. Family PROCELLARIIDAE Leach: Fulmars, Petrels, Prions and Shearwaters Procellariidae Leach, 1820: Eleventh room. In Synopsis Contents British Museum 17th Edition, London: 68 – Type genus Procellaria Linnaeus, 1758. Subfamilies Procellariinae and Fulmarinae and shearwater subgenera Ardenna, Thyellodroma and Puffinus (as recognised by Checklist Committee 1990) are not accepted here given the lack of agreement about to which subgenera some species should be assigned (e.g. -

Petrelsrefs V1.1.Pdf
Introduction I have endeavoured to keep typos, errors, omissions etc in this list to a minimum, however when you find more I would be grateful if you could mail the details during 2017 & 2018 to: [email protected]. Please note that this and other Reference Lists I have compiled are not exhaustive and are best employed in conjunction with other sources. Grateful thanks to Killian Mullarney and Tom Shevlin (www.irishbirds.ie) for the cover images. All images © the photographers. Joe Hobbs Index The general order of species follows the International Ornithologists' Union World Bird List (Gill, F. & Donsker, D. (eds.) 2017. IOC World Bird List. Available from: http://www.worldbirdnames.org/ [version 7.3 accessed August 2017]). Version Version 1.1 (August 2017). Cover Main image: Bulwer’s Petrel. At sea off Madeira, North Atlantic. 14th May 2012. Picture by Killian Mullarney. Vignette: Northern Fulmar. Great Saltee Island, Co. Wexford, Ireland. 5th May 2008. Picture by Tom Shevlin. Species Page No. Antarctic Petrel [Thalassoica antarctica] 12 Beck's Petrel [Pseudobulweria becki] 18 Blue Petrel [Halobaena caerulea] 15 Bulwer's Petrel [Bulweria bulweri] 24 Cape Petrel [Daption capense] 13 Fiji Petrel [Pseudobulweria macgillivrayi] 19 Fulmar [Fulmarus glacialis] 8 Giant Petrels [Macronectes giganteus & halli] 4 Grey Petrel [Procellaria cinerea] 19 Jouanin's Petrel [Bulweria fallax] 27 Kerguelen Petrel [Aphrodroma brevirostris] 16 Mascarene Petrel [Pseudobulweria aterrima] 17 Parkinson’s Petrel [Procellaria parkinsoni] 23 Southern Fulmar [Fulmarus glacialoides] 11 Spectacled Petrel [Procellaria conspicillata] 22 Snow Petrel [Pagodroma nivea] 14 Tahiti Petrel [Pseudobulweria rostrata] 18 Westland Petrel [Procellaria westlandica] 23 White-chinned Petrel [Procellaria aequinoctialis] 20 1 Relevant Publications Beaman, M. -

Importance of Ice Algal Production for Top Predators: New Insights Using Sea-Ice Biomarkers
Vol. 513: 269–275, 2014 MARINE ECOLOGY PROGRESS SERIES Published October 22 doi: 10.3354/meps10971 Mar Ecol Prog Ser FREEREE ACCESSCCESS Importance of ice algal production for top predators: new insights using sea-ice biomarkers A. Goutte1,2,*, J.-B. Charrassin1, Y. Cherel2, A. Carravieri2, S. De Grissac2, G. Massé1,3 1LOCEAN/IPSL — UMR 7159 Centre National de la Recherche Scientifique/Université Pierre et Marie Curie/ Institut de Recherche pour le Développement/Museum National d’Histoire Naturelle, 75005 Paris, France 2Centre d’Etudes Biologiques de Chizé, Centre National de la Recherche Scientifique, UPR 1934, 79360 Beauvoir sur Niort, France 3Centre National de la Recherche Scientifique and Université Laval, UMI 3376, Takuvik, Québec G1V 0A6, Canada ABSTRACT: Antarctic seals and seabirds are strongly dependent on sea-ice cover to complete their life history. In polar ecosystems, sea ice provides a habitat for ice-associated diatoms that en - sures a substantial production of organic matter. Recent studies have presented the potential of highly branched isoprenoids (HBIs) for tracing carbon flows from ice algae to higher-trophic-level organisms. However, to our knowledge, this new method has never been applied to sub-Antarctic species and Antarctic seals. Moreover, seasonal variations in HBI levels have never been investi- gated in Antarctic predators, despite a likely shift in food source from ice-derived to pelagic organic matter after sea-ice retreat. In the present study, we described HBI levels in a community of seabirds and seals breeding in Adélie Land, Antarctica. We then validated that sub-Antarctic seabirds had lower levels of diene, a HBI of sea-ice diatom origin, and higher levels of triene, a HBI of phytoplanktonic origin, compared with Antarctic seabirds. -

The Incidence, Functions and Ecological Significance of Petrel Stomach Oils
84 PROCEEDINGS OF THE NEW ZEALAND ECOLOGICAL SOCIETY, VOL. 24, 1977 THE INCIDENCE, FUNCTIONS AND ECOLOGICAL SIGNIFICANCE OF PETREL STOMACH OILS JOHN WARHAM Department of Zoology, University of Canterbury, Chrb;tchurch SUMMARY: Recent research into the origins and compositions of the stomach oils unique to sea~birds of the order Procellariifonnes is reviewed. The sources of these oils, most of which contain mainly wax esters and/or trigIycerides, is discussed in relation to the presence of such compounds in the marine environment. A number of functions are proposed as the ecological roles of the oils, including their use as slowIy~mobilisable energy and water reserves for adults and chicks and as defensive weaponry for surface-nesting species. Suggestions are made for further research, particularly into physiological and nutritional aspects. INTRODUCTION their table, a balm for their wounds, and a medicine Birds of the Order Procellariiforrnes (albatrosses, for their distempers." In New Zealand, Travers and fuJmars, shearwaters and other petrels) are peculiar Travers (1873) described how the Chatham Island in being able to store oil in their large, glandular and Morioris held young petrels over their mouths and very distensible fore-guts or proventriculi.. All petrel allowed the oil to drain directly into them. In some spedes so far examined, with the significant excep- years the St Kildans exported part of thei.r oil har~ tion of the diving petrels, Fam. Pelecanoididae, have vest, as the Australian mutton-birders stilI do with been found to contain oil at various times. The oi.1 oil from the chicks of P. tenuirostris. This has been occurs in both adults and chicks, in breeders and used as a basis for sun~tan lotions, but most nowa- non~breeders, and in birds taken at sea and on land. -

Cape Petrel (Southern)
RECOVERY OUTLINE Cape Petrel (southern) 1 Family Procellariidae 2 Scientific name Daption capense capense Linnaeus, 1758 3 Common name Cape Petrel (southern) 4 Conservation status Australian breeding population Vulnerable: D2 Population visiting Australian territory Least Concern 5 Reasons for listing The Australian population breeds at a single location (Vulnerable: D2). Globally the species is listed as Least Concern. Site fidelity is high, so it is assumed that the immigration rate is low. The national status of the breeding population is therefore determined independently of the global status (as per Gärdenfors et al., 1999). Australian breeding Estimate Reliability colonies Extent of occurrence 5,000,000 km2 low trend stable high 2 Area of occupancy 20 km medium 10 Threats trend stable low There are no imminent threats. At sea, some birds are No. of breeding birds 1,000 low likely to be caught on long-lines, but the subspecies is trend stable low usually displaced at feeding areas by larger scavengers, No. of sub-populations 1 high such as albatrosses and giant-petrels. The accidental Generation time 15 years low introduction of rats or cats is a potential threat. Global population share < 1 % high 11 Information required Level of genetic exchange low low None. 6 Infraspecific taxa D. c. australe (New Zealand region) is Least Concern. 12 Recovery objectives 12.1 Maintenance of the existing population. 7 Past range and abundance In Australian waters, breeding on Heard I. (Downes et 13 Actions completed or under way al., 1959, Woehler, 1991). Extralimitally, breeding on 13.1 Population is monitored opportunistically. islands throughout Southern Ocean. -

Appendix D New Zealand Conservation Status And
Appendix D New Zealand conservation status and International Union for Conservation of Nature (IUCN) ‘Red List’ threat classification of seabirds mentioned in the report and additionally in Paragraphs 12, 23 and 24 of my evidence. New Zealand conservation status is taken from Robertson et al. (2013), and ‘Red List’ classification from http://www.iucnredlist.org/, where further information regarding categories and criteria employed by the two systems can be found. Taxa marked * were referred to in general, non-specific terms in the report (for example, northern royal albatross here was referred to as ‘unidentified royal albatross’ in the report), but are included here as species for completeness. Taxa are listed in the order they appear in the report, then in the order they appear in Paragraphs 12, 23 and 24 of my evidence. Taxa NZ Conservation Status Red List classification Wandering albatross Migrant Vulnerable Northern royal albatross* At Risk – Naturally Uncommon Endangered Southern royal albatross* At Risk – Naturally Uncommon Vulnerable Antipodean albatross Threatened – Nationally Critical Vulnerable Gibson’s albatross Threatened – Nationally Critical Vulnerable Black-browed albatross Coloniser Near Threatened Campbell albatross At Risk - Naturally Uncommon Vulnerable Grey-headed albatross Threatened – Nationally Vulnerable Endangered Southern Buller’s albatross At Risk - Naturally Uncommon Near Threatened Salvin’s albatross Threatened – Nationally Critical Vulnerable Northern giant petrel* At Risk - Naturally Uncommon Least Concern -

Densities of Antarctic Seabirds at Sea and the Presence of the Krill Eupha Usia Superba
DENSITIES OF ANTARCTIC SEABIRDS AT SEA AND THE PRESENCE OF THE KRILL EUPHA USIA SUPERBA BRYAN S. OBST Departmentof Biology,University of California,Los Angeles, California 90024 USA ABSTRACT.--Theantarctic krill Euphausiasuperba forms abundant,well-organized schools in the watersoff the AntarcticPeninsula. Mean avian densityis 2.6 timesgreater in waters where krill schoolsare present than in waters without krill schools.Seabird density is a good predictorof the presenceof krill. Seabirddensity did not correlatewith krill density or krill schooldepth. Disoriented krill routinely were observedswimming near the surface above submergedschools, providing potential prey for surface-feedingbirds. Responsesof seabird speciesto the distribution of krill schoolsvaried. The small to me- dium-sizeprocellariiform species were the best indicatorsof krill schools;large procellari- iforms and coastalspecies were poor indicators.Pygoscelis penguins occurredat high den- sitiesonly in the presenceof krill schools.These responses are consistentwith the constraints imposedby the metabolicrequirements and reproductivestrategies of eachof thesegroups. Krill schoolswere detectednear the seasurface throughout the day. Correlationsbetween seabirddensity and the presenceof krill during daylight hourssuggest that diurnal foraging is important to the seabirdsof this region. Received19 December1983, accepted4 December 1984. RELATIVELY little is known about the factors birds depend on directly or indirectly for food influencing the distribution of seabirdsin the (Haury et al. 1978). These observationssuggest marine habitat. The past decade has produced that relatively small-scalephenomena, such as a number of studiesattempting to correlatepat- local concentrationsof prey, may be of major terns of avian abundance and distribution with importance in determining the patterns of sea- physical featuresof the oceansuch as currents bird distribution within the broad limits set by and convergences,water masses,and temper- featuresof the physical ocean. -
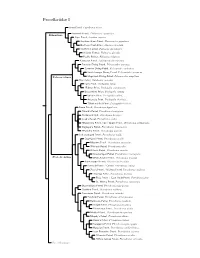
Procellariidae Species Tree
Procellariidae I Snow Petrel, Pagodroma nivea Antarctic Petrel, Thalassoica antarctica Fulmarinae Cape Petrel, Daption capense Southern Giant-Petrel, Macronectes giganteus Northern Giant-Petrel, Macronectes halli Southern Fulmar, Fulmarus glacialoides Atlantic Fulmar, Fulmarus glacialis Pacific Fulmar, Fulmarus rodgersii Kerguelen Petrel, Aphrodroma brevirostris Peruvian Diving-Petrel, Pelecanoides garnotii Common Diving-Petrel, Pelecanoides urinatrix South Georgia Diving-Petrel, Pelecanoides georgicus Pelecanoidinae Magellanic Diving-Petrel, Pelecanoides magellani Blue Petrel, Halobaena caerulea Fairy Prion, Pachyptila turtur ?Fulmar Prion, Pachyptila crassirostris Broad-billed Prion, Pachyptila vittata Salvin’s Prion, Pachyptila salvini Antarctic Prion, Pachyptila desolata ?Slender-billed Prion, Pachyptila belcheri Bonin Petrel, Pterodroma hypoleuca ?Gould’s Petrel, Pterodroma leucoptera ?Collared Petrel, Pterodroma brevipes Cook’s Petrel, Pterodroma cookii ?Masatierra Petrel / De Filippi’s Petrel, Pterodroma defilippiana Stejneger’s Petrel, Pterodroma longirostris ?Pycroft’s Petrel, Pterodroma pycrofti Soft-plumaged Petrel, Pterodroma mollis Gray-faced Petrel, Pterodroma gouldi Magenta Petrel, Pterodroma magentae ?Phoenix Petrel, Pterodroma alba Atlantic Petrel, Pterodroma incerta Great-winged Petrel, Pterodroma macroptera Pterodrominae White-headed Petrel, Pterodroma lessonii Black-capped Petrel, Pterodroma hasitata Bermuda Petrel / Cahow, Pterodroma cahow Zino’s Petrel / Madeira Petrel, Pterodroma madeira Desertas Petrel, Pterodroma