Liquid-To-Glass Transformation Simply Because This Is a Standalone Phenomenon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Niobium Doped BGO Glasses: Physical, Thermal and Optical Properties
IOSR Journal of Applied Physics (IOSR-JAP) e-ISSN: 2278-4861. Volume 3, Issue 5 (Mar. - Apr. 2013), PP 80-87 www.iosrjournals.org Niobium doped BGO glasses: Physical, Thermal and Optical Properties Khair-u-Nisa1, Ejaz Ahmed2, M. Ashraf Chaudhry3 1,2,3Department of Physics, Bahauddin Zakariya University, Multan (60800), Pakistan. Abstract: IR- transparent niobium substituted heavy metal oxide glass system in formula composition (100-x) BGO75- xNb2O5; 5 ≤ x ≤ 25 was fabricated by quenching and press molding technique. Glassy state was confirmed by XRD. Density ρexp varied 6.381 g/cc-7.028 g/cc ±0.06%. Modifying behavior of Nb2O5 was corroborated by rate of increase in theoretical volume Vth, measured volume Vexp and oxygen molar volume -2 5+ 4+ VMO . Nb had larger cation radius and greater polarizing strength as compared to Ge ions. It replaced 4+ o Ge sites introducing more NBOs in the network. Transformation temperatures Tg, Tx and Tp1 were 456 -469 C ±2 oC, 516 -537 oC ±2 oC and 589 -624 oC ±2 oC respectively. In the range from room temperature to 400 oC -6 -1 -6 -1 the coefficient of linear thermal expansion α was 5.431±0.001*10 K to 7.333±0.001*10 K . ΔT = Tx-Tg and ΔTP1 = TP1-Tg varied collinearly with increase in niobium concentration and revealed thermal stability against devitrification. The direct bandgap Eg values lay in 3.24 -2.63 eV ±0.01 eV range and decreased due to impurity states of Nb5+ within the forbidden band. Mobility edges obeyed Urbach law verifying amorphousness of the compositions. -
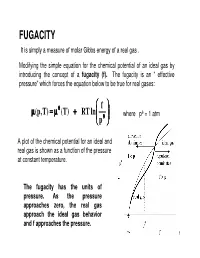
FUGACITY It Is Simply a Measure of Molar Gibbs Energy of a Real Gas
FUGACITY It is simply a measure of molar Gibbs energy of a real gas . Modifying the simple equation for the chemical potential of an ideal gas by introducing the concept of a fugacity (f). The fugacity is an “ effective pressure” which forces the equation below to be true for real gases: θθθ f µµµ ,p( T) === µµµ (T) +++ RT ln where pθ = 1 atm pθθθ A plot of the chemical potential for an ideal and real gas is shown as a function of the pressure at constant temperature. The fugacity has the units of pressure. As the pressure approaches zero, the real gas approach the ideal gas behavior and f approaches the pressure. 1 If fugacity is an “effective pressure” i.e, the pressure that gives the right value for the chemical potential of a real gas. So, the only way we can get a value for it and hence for µµµ is from the gas pressure. Thus we must find the relation between the effective pressure f and the measured pressure p. let f = φ p φ is defined as the fugacity coefficient. φφφ is the “fudge factor” that modifies the actual measured pressure to give the true chemical potential of the real gas. By introducing φ we have just put off finding f directly. Thus, now we have to find φ. Substituting for φφφ in the above equation gives: p µ=µ+(p,T)θ (T) RT ln + RT ln φ=µ (ideal gas) + RT ln φ pθ µµµ(p,T) −−− µµµ(ideal gas ) === RT ln φφφ This equation shows that the difference in chemical potential between the real and ideal gas lies in the term RT ln φφφ.φ This is the term due to molecular interaction effects. -

Lecture 4 (Pdf)
GFD 2006 Lecture 4: Interfacial instability in two-component melts Grae Worster; notes by Shane Keating and Takahide Okabe March 15, 2007 So far, we have looked at some of the fundamentals associated with solidification of pure melts. When we try to solidify a solution of two or more components, salt and water, for example, the character of the solidification changes considerably. In particular, the presence of salt can depress the temperature at which ice and salt water can coexist in thermal equilibrium. This has an important consequence for the growth of sea ice: unless there is some other mechanism for the transport of the salt field, such as convection, the growth of the ice is limited by the rate at which excess salt can diffuse away from the interface. Finally, we will discuss the morphological instability in two-component melts. We shall see that the solute field is destabilizing and can give rise to morphological instability even when the liquid phase is not initially supercooled. 1 Two-component melts 1.1 A simple demonstration We shall begin with a simple demonstration. Crushed ice at 0◦C is placed in a cup with a thermometer. We add a handful of salt at room temperature and stir briskly. The ice begins to melt, but what happens to the temperature? We notice that there is some melt water in the cup, which helps bring the ice and salt into contact, and see a fairly rapid decrease in the temperature measured by the thermometer: after a few minutes, it reads almost 10 C. -

A Study of the Equilibrium Diagrams of the Systems, Benzene-Toluene and Benzene-Ethylbenzene
Loyola University Chicago Loyola eCommons Master's Theses Theses and Dissertations 1940 A Study of the Equilibrium Diagrams of the Systems, Benzene- Toluene and Benzene-Ethylbenzene John B. Mullen Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_theses Part of the Chemistry Commons Recommended Citation Mullen, John B., "A Study of the Equilibrium Diagrams of the Systems, Benzene-Toluene and Benzene- Ethylbenzene" (1940). Master's Theses. 299. https://ecommons.luc.edu/luc_theses/299 This Thesis is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Master's Theses by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1940 John B. Mullen A S'l'IJDY OF THE EQUTI.IBRIUM DIAGRAMS OF THE SYSTEMS, BENZENE-TOLUENE AND BENZENE-~ENZENE By John B. Mullen Presented in partial :f'u.J.tilment at the requirements :f'or the degree o:f' Master o:f' Science, Loyola University, 1940. T.ABLE OF CONTENTS Page Acknowledgment 1i Vita iii Introduction 1 I. Review of Literature 2 II. Apparatus and its Calibration 3 III. Procedure am Technique 6 IV. Observations on the System Benzene-Toluene 9 V. Observations on the System Benzene-Ethylbenzene 19 VI. Equilibrium Diagrams of the Systems 30 Recommendations for Future Work 34 Bibliography 35 ACKNOWLEOOMENT The au1hor wishes to acknowledge with thanks the invaluable assistance, suggestions, and cooperation offered by Dr. -

Fluidized Bed Chemical Vapor Deposition of Zirconium Nitride Films
INL/JOU-17-42260-Revision-0 Fluidized Bed Chemical Vapor Deposition of Zirconium Nitride Films Dennis D. Keiser, Jr, Delia Perez-Nunez, Sean M. McDeavitt, Marie Y. Arrieta July 2017 The INL is a U.S. Department of Energy National Laboratory operated by Battelle Energy Alliance INL/JOU-17-42260-Revision-0 Fluidized Bed Chemical Vapor Deposition of Zirconium Nitride Films Dennis D. Keiser, Jr, Delia Perez-Nunez, Sean M. McDeavitt, Marie Y. Arrieta July 2017 Idaho National Laboratory Idaho Falls, Idaho 83415 http://www.inl.gov Prepared for the U.S. Department of Energy Under DOE Idaho Operations Office Contract DE-AC07-05ID14517 Fluidized Bed Chemical Vapor Deposition of Zirconium Nitride Films a b c c Marie Y. Arrieta, Dennis D. Keiser Jr., Delia Perez-Nunez, * and Sean M. McDeavitt a Sandia National Laboratories, Albuquerque, New Mexico 87185 b Idaho National Laboratory, Idaho Falls, Idaho 83401 c Texas A&M University, Department of Nuclear Engineering, College Station, Texas 77840 Received November 11, 2016 Accepted for Publication May 23, 2017 Abstract — – A fluidized bed chemical vapor deposition (FB-CVD) process was designed and established in a two-part experiment to produce zirconium nitride barrier coatings on uranium-molybdenum particles for a reduced enrichment dispersion fuel concept. A hot-wall, inverted fluidized bed reaction vessel was developed for this process, and coatings were produced from thermal decomposition of the metallo-organic precursor tetrakis(dimethylamino)zirconium (TDMAZ) in high- purity argon gas. Experiments were executed at atmospheric pressure and low substrate temperatures (i.e., 500 to 550 K). Deposited coatings were characterized using scanning electron microscopy, energy dispersive spectroscopy, and wavelength dis-persive spectroscopy. -

Phase Diagrams
Module-07 Phase Diagrams Contents 1) Equilibrium phase diagrams, Particle strengthening by precipitation and precipitation reactions 2) Kinetics of nucleation and growth 3) The iron-carbon system, phase transformations 4) Transformation rate effects and TTT diagrams, Microstructure and property changes in iron- carbon system Mixtures – Solutions – Phases Almost all materials have more than one phase in them. Thus engineering materials attain their special properties. Macroscopic basic unit of a material is called component. It refers to a independent chemical species. The components of a system may be elements, ions or compounds. A phase can be defined as a homogeneous portion of a system that has uniform physical and chemical characteristics i.e. it is a physically distinct from other phases, chemically homogeneous and mechanically separable portion of a system. A component can exist in many phases. E.g.: Water exists as ice, liquid water, and water vapor. Carbon exists as graphite and diamond. Mixtures – Solutions – Phases (contd…) When two phases are present in a system, it is not necessary that there be a difference in both physical and chemical properties; a disparity in one or the other set of properties is sufficient. A solution (liquid or solid) is phase with more than one component; a mixture is a material with more than one phase. Solute (minor component of two in a solution) does not change the structural pattern of the solvent, and the composition of any solution can be varied. In mixtures, there are different phases, each with its own atomic arrangement. It is possible to have a mixture of two different solutions! Gibbs phase rule In a system under a set of conditions, number of phases (P) exist can be related to the number of components (C) and degrees of freedom (F) by Gibbs phase rule. -

Structural Transformation in Supercooled Water Controls the Crystallization Rate of Ice
Structural transformation in supercooled water controls the crystallization rate of ice. Emily B. Moore and Valeria Molinero* Department of Chemistry, University of Utah, Salt Lake City, UT 84112-0580, USA. Contact Information for the Corresponding Author: VALERIA MOLINERO Department of Chemistry, University of Utah 315 South 1400 East, Rm 2020 Salt Lake City, UT 84112-0850 Phone: (801) 585-9618; fax (801)-581-4353 Email: [email protected] 1 One of water’s unsolved puzzles is the question of what determines the lowest temperature to which it can be cooled before freezing to ice. The supercooled liquid has been probed experimentally to near the homogeneous nucleation temperature T H≈232 K, yet the mechanism of ice crystallization—including the size and structure of critical nuclei—has not yet been resolved. The heat capacity and compressibility of liquid water anomalously increase upon moving into the supercooled region according to a power law that would diverge at T s≈225 K,1,2 so there may be a link between water’s thermodynamic anomalies and the crystallization rate of ice. But probing this link is challenging because fast crystallization prevents experimental studies of the liquid below T H. And while atomistic studies have captured water crystallization3, the computational costs involved have so far prevented an assessment of the rates and mechanism involved. Here we report coarse-grained molecular simulations with the mW water model4 in the supercooled regime around T H, which reveal that a sharp increase in the fraction of four-coordinated molecules in supercooled liquid water explains its anomalous thermodynamics and also controls the rate and mechanism of ice formation. -

Neutron Star Cooling and Discuss the Main Issue: What We Can Learn About the Internal Structure of Neutron Stars by Confronting Theory and Observation
To appear in Ann. Rev. Astron. Astrophys. 2004 Neutron Star Cooling D. G. Yakovlev 1 and C. J. Pethick 2 1Ioffe Physical Technical Institute, Politekhnicheskaya 26, 194021 St.-Petersburg, Russia, e-mail: [email protected]ffe.ru 2NORDITA, The Nordic Institute for Theoretical Physics, Blegdamsvej 17, DK– 2100 Copenhagen Ø, Denmark, e-mail: [email protected] Abstract Observation of cooling neutron stars can potentially provide information about the states of matter at supernuclear densities. We review physical properties important for cooling such as neutrino emission processes and superfluidity in the stellar interior, surface envelopes of light elements due to accretion of matter and strong surface magnetic fields. The neutrino pro- cesses include the modified Urca process, and the direct Urca process for nucleons and exotic states of matter such as a pion condensate, kaon con- densate, or quark matter. The dependence of theoretical cooling curves on physical input and observations of thermal radiation from isolated neutron stars are described. The comparison of observation and theory leads to a unified interpretation in terms of three characteristic types of neutron stars: high-mass stars which cool primarily by some version of the direct Urca process; low-mass stars, which cool via slower processes; and medium-mass stars, which have an intermediate behavior. The related problem of thermal states of transiently accreting neutron stars with deep crustal burning of accreted matter is discussed in connection with observations of soft X-ray transients. arXiv:astro-ph/0402143v1 6 Feb 2004 1 INTRODUCTION Neutron stars are the most compact stars in the Universe. They have masses M ∼ 1.4 M⊙ and radii R ∼ 10 km, and they contain matter at supernuclear densities in their cores. -

Technical Glasses
Technical Glasses Physical and Technical Properties 2 SCHOTT is an international technology group with 130 years of ex perience in the areas of specialty glasses and materials and advanced technologies. With our highquality products and intelligent solutions, we contribute to our customers’ success and make SCHOTT part of everyone’s life. For 130 years, SCHOTT has been shaping the future of glass technol ogy. The Otto Schott Research Center in Mainz is one of the world’s leading glass research institutions. With our development center in Duryea, Pennsylvania (USA), and technical support centers in Asia, North America and Europe, we are present in close proximity to our customers around the globe. 3 Foreword Apart from its application in optics, glass as a technical ma SCHOTT Technical Glasses offers pertinent information in terial has exerted a formative influence on the development concise form. It contains general information for the deter of important technological fields such as chemistry, pharma mination and evaluation of important glass properties and ceutics, automotive, optics, optoelectronics and information also informs about specific chemical and physical character technology. Traditional areas of technical application for istics and possible applications of the commercial technical glass, such as laboratory apparatuses, flat panel displays and glasses produced by SCHOTT. With this brochure, we hope light sources with their various requirements on chemical to assist scientists, engineers, and designers in making the physical properties, have led to the development of a great appropriate choice and make optimum use of SCHOTT variety of special glass types. Through new fields of appli products. cation, particularly in optoelectronics, this variety of glass types and their modes of application have been continually Users should keep in mind that the curves or sets of curves enhanced, and new forming processes have been devel shown in the diagrams are not based on precision measure oped. -

Cooling Electrons in Semiconductor Devices: a Model of Evaporative Emission
PHYSICAL REVIEW B 75, 035316 ͑2007͒ Cooling electrons in semiconductor devices: A model of evaporative emission Thushari Jayasekera, Kieran Mullen, and Michael A. Morrison* Homer L. Dodge Department of Physics and Astronomy, The University of Oklahoma, 440 West Brooks Street, Norman, Oklahoma 73019-0225, USA ͑Received 4 April 2006; revised manuscript received 2 November 2006; published 11 January 2007͒ We discuss the theory of cooling electrons in solid-state devices via “evaporative emission.” Our model is based on filtering electron subbands in a quantum-wire device. When incident electrons in a higher-energy subband scatter out of the initial electron distribution, the system equilibrates to a different chemical potential and temperature than those of the incident electron distribution. We show that this re-equilibration can cause considerable cooling of the system. We discuss how the device geometry affects the final electron temperatures, and consider factors relevant to possible experiments. We demonstrate that one can therefore induce substantial electron cooling due to quantum effects in a room-temperature device. The resulting cooled electron population could be used for photodetection of optical frequencies corresponding to thermal energies near room temperature. DOI: 10.1103/PhysRevB.75.035316 PACS number͑s͒: 73.50.Lw, 73.23.Ϫb I. INTRODUCTION achieve electron cooling. We improve upon this result by switching to a “plus-junction” design, which improves the As electronic devices become smaller, they leave the re- cooling characteristics of the device. In Sec. IV we discuss gime of classical physics and enter the realm of quantum applications and realistic parameters for a device to cool physics. -

Exam3 Practice
Materials Science and Engineering Department MSE 360, Test #3 ID number _______________________________ Name: _____________________________________ No notes, books, or information stored in calculator memories may be used. Cheating will be punished severely. All of your work must be written on these pages and turned in. Constants, equations, and other data are given on the last page of the exam. _______________________________________________________________________________ Problem 1-18: 2 points each (Key:1C2B3B4B5A6D 7B8D9B10B11A12A 13A14A15A16B17A18A) 1. A regular solution will likely form an ordered atomic structure if: d.) Ω = 0 b.) Ω > 0 c.) Ω < 0 2. With increasing temperature and constant pressure, the Gibbs free energy of a single phase A) increase, B) decrease 3. During annealing, the grain #2 represented in the right figure will A) be stable B) grow C) shrink 4. The mechanism of diffusion for a dilute solute atom of small atomic radius compared to the host material would be best described by: a.) Substitutional diffusion b.) Interstitial diffusion c.) Vacancy diffusion d.) all of the above 5. When Lf/Tm > 4R, where the Lf is the latent heat of fusion, Tm is the melting temperature, the liquid/solid interface is A) Smooth, B) Rough 6. The inhomogeneous nucleation is easier than the homogeneous nucleation because the inhomogeneous nucleation A. Has a critical nucleus with smaller diameter B. Has a critical nucleus with smaller volume C. Has a critical nucleus with smaller critical Gibbs free energy D. Both B and C 7. For heterogeneous nucleation in the grain interior, grain boundaries, grain edges and grain corners, their critical energy barriers can be described as A. -

The Correlation Between Reduced Glass Transition Temperature and Glass Forming Ability of Bulk Metallic Glasses Z.P
http://www.paper.edu.cn Scripta mater. 42 (2000) 667–673 www.elsevier.com/locate/scriptamat THE CORRELATION BETWEEN REDUCED GLASS TRANSITION TEMPERATURE AND GLASS FORMING ABILITY OF BULK METALLIC GLASSES Z.P. Lu, H. Tan, Y. Li* and S.C. Ngϩ Department of Materials Science, National University of Singapore, Singapore 119260, People’s Republic of China ϩDepartment of Physics, National University of Singapore, Singapore 119260, People’s Republic of China (Received October 4, 1999) (Accepted November 9, 1999) Keywords: Differential thermal analysis (DTA); Metallic glasses; Melt-spinning; Glass forming ability (GFA) 1. Introduction Based on theoretical work on crystal nucleation in undercooled liquid metals, Turnbull [1] proposed that the glass-forming tendency should increase with the reduced glass transition temperature, Trg, which was defined by Tg/Tl. Here, Tg is the glass transition temperature and Tl is the liquidus temperature. Later work of Uhlmann, Davies and others on the crystal nucleation further identified this dimension- less parameter as a crucial figure of merit in determining glass forming ability (GFA) [2–4]. There are many reported values of Trg in the literature [5], but unfortunately most of them were calculated using Tg/Tm (Tm: onset melting point) with minimal report of Tg/Tl [6,7]. Tg/Tm and Tg/Tl were used for evaluation of Trg interchangeably by most of researchers. Also many used crystallization temperature Tx instead of Tg, since for most of the metallic glass, these two were regarded as the same [3]. In this study, onset temperature (solidus), Tm and offset temperature (liquidus) Tl of melting of a series of bulk glass forming alloys based on Zr, La, Mg, Pd and rare-earth elements were measured by studying systematically the melting behaviour of these alloys using DTA or DSC.