Phytochemical, Antimicrobial and Bio-Active Component Analysis of Platycerium Superbum (L.) Methanolic Extract
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spring Australian Plants Society Armidale & District Group
APS Armidale 2015_3 Spring Australian Plants Society Armidale & District Group PO Box 735 Armidale NSW 2350 Crowea exalata ssp magnifolia image by Maria Hitchcock Web: http://www.aps-armidale.org.au e-mail: [email protected] Spring Edition 2015 - 3 In this issue: Office bearers for 2015 ...p.2 President’s Message ...p.3 From little things, big things grow ...p.3 Garden Visits Report: Saturday 23 May …p.4 National Correa Collection Web Site ...p.5 Soltice Event Report: Sunday 21 June …p.6 Spring in Autumn and Winter ...p.6 Possum Magic in Armidale …p.7 A tale of “Pinky” and Others ...p.8 Mole Station Weekend ...p.9 A Pleasant Horticultural surprise ...p.10 Frost Likelihood Audit ...p.11 Photo: Leptospermum spectabile from the Mulquiney Garden (not in situ) photo C. Mulquiney. Frost Likelihood Audit Form ...p.14 Contact Us: Armidale & District Group PO Box 735, Armidale NSW 2350 President: Phil Rose Ph. 6775 3767 [email protected] Secretary: Helen Schwarz Ph. 6772 1584 [email protected] Treasurer: Carole Fullalove [email protected] From the newsletter editor: Dear members, this is your newsletter and all articles, snippets and photos are welcome. The issue deadlines are 2 weeks before the Business Meeting. Articles will be included based on a FIRST COME basis. Please send your articles, snippets etc to me. Page 1 APS Armidale 2015_3 Spring GROUP INFORMATION The Armidale and District Group of APS--NSW started on 6th August, 1977 as the New England Group of the Society for Growing Australian Plants. It has been running continuously since that time with a couple of name changes. -

Review of Selected Literature and Epiphyte Classification
--------- -- ---------· 4 CHAPTER 1 REVIEW OF SELECTED LITERATURE AND EPIPHYTE CLASSIFICATION 1.1 Review of Selected, Relevant Literature (p. 5) Several important aspects of epiphyte biology and ecology that are not investigated as part of this work, are reviewed, particularly those published on more. recently. 1.2 Epiphyte Classification and Terminology (p.11) is reviewed and the system used here is outlined and defined. A glossary of terms, as used here, is given. 5 1.1 Review of Selected, Relevant Li.terature Since the main works of Schimper were published (1884, 1888, 1898), particularly Die Epiphytische Vegetation Amerikas (1888), many workers have written on many aspects of epiphyte biology and ecology. Most of these will not be reviewed here because they are not directly relevant to the present study or have been effectively reviewed by others. A few papers that are keys to the earlier literature will be mentioned but most of the review will deal with topics that have not been reviewed separately within the chapters of this project where relevant (i.e. epiphyte classification and terminology, aspects of epiphyte synecology and CAM in the epiphyt~s). Reviewed here are some special problems of epiphytes, particularly water and mineral availability, uptake and cycling, general nutritional strategies and matters related to these. Also, all Australian works of any substance on vascular epiphytes are briefly discussed. some key earlier papers include that of Pessin (1925), an autecology of an epiphytic fern, which investigated a number of factors specifically related to epiphytism; he also reviewed more than 20 papers written from the early 1880 1 s onwards. -

Fern News 88
ASSOCIATION 0f Mag; MW 88 ISSN 0811-5311 DATE ‘- MARCH 2000 ‘30.}? ********************************************************************************** LEADER Peter Hind, 41 Miller Street, Mount Druitt. N. S. W. 2770 SECRETARY: Vacant TREASURER: Joan Moore, 2 Gannet Street, Gladesville. N. S.W. 21 11 NEWSLETTER EDITOR: Mike Healy, 272 Humffray St Nth Ballarat. Vic 3350 E- mail address: nhealy@telstra easymail. corn. au> (N. B. It 13 n n_otmhea1y) SPORE BANK: Barry White, 24 Ruby Street, West Essendon Vic. 3040 ********************************************************************************** REMINDER YEAR 2000 SUBSCRIPTIONS ARE NOW OVERDUE. Thanks to all who have already forwarded their fees to J oan Moore. Could those who have not yet done so please forward your $5 to loan at the above address immediately ‘FERNS 0F TASMANIA’ by Michael Garrett, Joan has one copy for sale. Anyone interested, please contact Joan Moore. AUSTRALIAN PLANTS & VICTORIAN FERN GROWER CONTRIBUTE TO PRINCE CHARLE’S 50TH BIRTHDAY. The following article from the British Pteridological Society Bulletin (Vol. 5 - Number 4 — 1999) illustrates The Prince of Wales love of ferns: The Society engaged Victorian Fem grower, Chris Goudey, to provide Leptopteris superba — a ‘special’ NZ. fern to further personalise their gift hit the occasion. Creative table decorations used at the Prince’s birthday party may be of interest. FERN GIFT FOR PRINCE OF WALES AT HIGHGROVE by Martin Rickard Members will remember that we circulated a request for ferns to be given to His Royal Highness The Prince of Wales on the occasion of his fiftieth birthday. The momentous birthday was in November 1998 but by the nature of organising a gift from quite a few members, the ferns were not gathered together until the spring of 1999, a good season For delivery The coilectionincluded many rare and uncommon cultivars including: large trunked specimens of Dicksania antarctica, D. -

Polypodiophyta): a Global Assessment of Traits Associated with Invasiveness and Their Distribution and Status in South Africa
Terrestrial alien ferns (Polypodiophyta): A global assessment of traits associated with invasiveness and their distribution and status in South Africa By Emily Joy Jones Submitted in fulfilment of the requirements for the degree Master of Science in the Faculty of Science at the Nelson Mandela University April 2019 Supervisor: Dr Tineke Kraaij Co-Supervisor: Dr Desika Moodley Declaration I, Emily Joy Jones (216016479), hereby indicate that the dissertation for Master of Science in the Faculty of Science is my own work and that it has not previously been submitted for assessment or completion of any postgraduate qualification to another University or for another qualification. _______________________ 2019-03-11 Emily Joy Jones DATE Official use: In accordance with Rule G4.6.3, 4.6.3 A treatise/dissertation/thesis must be accompanied by a written declaration on the part of the candidate to the effect that it is his/her own work and that it has not previously been submitted for assessment to another University or for another qualification. However, material from publications by the candidate may be embodied in a treatise/dissertation/thesis. i Table of Contents Abstract ...................................................................................................................................... i Acknowledgements ................................................................................................................ iii List of Tables ........................................................................................................................... -

Platycerium Night — San Diego Fern Society, Jan. 2011 Submitted by Kathie Russell to Start the Fern Growing Year in San Diego, Califor- for Years
Volume 38 Number 2 April-May 2011 Editors: Joan Nester-Hudson and David Schwartz Platycerium Night — San Diego Fern Society, Jan. 2011 submitted by Kathie Russell To start the fern growing year in San Diego, Califor- for years. He watched his grandfather grow them, and nia, we traditionally have a Platycerium Night in Janu- now Don grows many species and cultivars and has al- ary for our Fern Society. It seems that the staghorn ferns ways been willing to share his experiences. He has also are growing at this time of year, and it can be a great observed plants in nature growing in Australia, Thai- time to divide plants and remount the “pups.” land, Cambodia, Laos, and the Philippines. Don Callard, who lives in Del Mar a few blocks from Platyceriums are found in tropical and subtropical ar- the Pacific Ocean, has grown Platyceriums in his yard eas of the Old World, with just one species, P. andinum, Fig. 1. Several Platyceriums including the P. superbum, about midway on display board (close-up shown in Fig. 2). These plants were displayed at the San Diego Fern Society Show in August 2010 and all were grown in southern California. photo by Kathie Russell Fiddlehead Forum u April-May 2011 u Page 9 in South America. Certain plants such as P. wallichi are known to have AFS OFFICERS a dormant period during the dry season. When water is provided, San PRESIDENT-: Michael D. Windham, Department of Biology, Duke University, Box 90338, Durham, NC Diego has a good climate for all but the most cold-sensitive plants. -

A.N.P.S.A. Fern Study Group Newsletter Number 119
A.N.P.S.A. Fern Study Group Newsletter Number 119 ISSN 1837-008X DATE : March, 2010 LEADER : Peter Bostock, PO Box 402, KENMORE , Qld 4069. Tel. a/h: 07 32026983, mobile: 0421 113 955; email: [email protected] TREASURER : Dan Johnston, 9 Ryhope St, BUDERIM , Qld 4556. Tel 07 5445 6069, mobile: 0429 065 894; email: [email protected] NEWSLETTER EDITOR : Dan Johnston, contact as above. SPORE BANK : Barry White, 34 Noble Way, SUNBURY , Vic. 3429 From the editor Thanks to Dot and Merle for their meeting reports, to Claire and Wendy for articles and to Barry for the spore list. As this newsletter was looking rather thin, I have included some material relating to Lord Howe Island, which Wendy and I have recently visited. This is only a very small sample of the wonderful range of ferns there. Program for South-east Queensland Region Dan Johnston Sunday, 7 th March, 2010. Excursion to Bryces Road, Brisbane Forest Park. Meet at 9:30am on the roadside near Camp Constable (between Mt Glorious Cafe and entrance road to Maiala National Park.) Weather is likely to be feral, so bring raingear! Saturday, 1 st May, 2010 to Monday, 3 rd May, 2010. Excursion concentrating on the Dorrigo area of Northern NSW. Current intentions are to seek motel accommodation in Coffs Harbour. Please register interest with Peter by phone or email as soon as possible. Sunday, 6 th June, 2010. Meet at 9:30am at Claire Shackel’s place, 19 Arafura St, Upper Mt Gravatt. Subject: Fern propagation. Sunday, 4 th July, 2010. -

Fern News 97
ASSOCIATION Of 96M???” m9¢¢§£¢ m2, “W97 ISSN 0811-5311 DATE JUNEJW 6-0.2»- $- ****************************************************************************** LEADER Peter Hind, 41 Miller Street, Mount Druitt. N. S. W. 2770 SECRETARY: TREASURER: R011 Wilkins, 188b Beecroft Rd., Cheltenham NSW 2119 E—mail: [email protected] NEWSLETTER ED1TOR:Mike Healy, 272 Humffray St. Nth, Ballarat. Vic. 3350 E-mail address: [email protected] SPORE BANK: Barry White, 24 Ruby Street; West Essendon. Vic. 3040 **********************************fi***************************************** PLEASE NOTE NEW E-MAIL ADDRESSES FOR TREASURER & NEWSLETTER EDITOR Please ensuxe future e-mail is directed to these addresses. Also anything sent afler the 11th May has not be received *********************************************** LIFE MEMBERSHIP ACKNOWLEDGES MEMBER’S SUPPORT TO FERN STUDY GRQUP The following correspondence was received from Peter Hind for inclusion in the newsletter. 21/02/ 2002 Dr. Calder Chaffey 'Red Fox", 13 Acacia St, Wollongbar, NSW 2477 Dear Dr Chaffey, At the last meeting of the Sydney ASGAP Fern Study Group it was unanimously agreed that you should be given Life Membership of the Fern Study Group We feel sure that other regional groups and individual members of the Fern Study Group would enthusiastically support the decision This is firstly 1n recogmtion of your major work on growing Australian ferns and the publication of your book "Australian Fems. Growing Them Successfully". It's a volume that gives many members pride to have been associated with in some small way. Secondly, your financial contributions to the Group by way of a share of the royalties from the book, have put the finances of the Group in a very sound position thus ensun'ng that the Group will be viable well into the future. -

1- 1; Lssn 0811-5311 DATE - = JUNE, 1986
7 i' I 1- 1; lSSN 0811-5311 DATE - = JUNE, 1986. d .Q.K* b* UREGISTERED BY AUSTRALIA POST 1 PUBLICATION NUMBER NBH 3809," r: 11 , -,'':I LEADER : Phyll. Brown, 254 Edgar Street, Condell Park. 2200 S EC RETARY : Moreen Woollett, 3 Currawang Plce., Como West 2226 HOE. TREASURER: Margaret Olde , 1 38 Fowl er Road, Illawong. 2234. SPORE BANK: Sylvia Garlick, 3 Valley view Cres. , Engadine , 2233. Dear Members, I can't think of a better time to start writing the news- letter than when listening to and watching the beautiful steady rain. It is hoped the rain is spreading far and wide to areas -I --- where it is so badly needed. -I -= 1. The usual group of members enjoyed the day at Lawson in the Blue Mountains and also when planting ferns in an area of the Joseph Banks garden at Kareela. Over the past year and a half we have become friends with Jan and Noel Laity and their daughter Anne, our chief ticket seller. They have been very active members, The Laitys are return- ing to their home state of Victoria where they have purchased a bakery business at Colac. We wish them $he very best of luck in their new venture. I want to specially thank Jan and Noel for their help at the Exhibition last year in the construction of the fern exhibition, selling tickets and claening up afterwards. Jan has indicated she hopes to to be back for this years show. Ye are making a very big effort thie ycar with three prizes in the raffle and all proceeds will go to Burrnedong Arboretum. -

The Emerging Weed Challenge of Managing Native Plant Species: What Are We Doing in New South Wales?
Nineteenth Australasian Weeds Conference The emerging weed challenge of managing native plant species: what are we doing in New South Wales? Stephen B. Johnson New South Wales Department of Primary Industries, Locked Bag 6006, Orange, NSW 2800, Australia ([email protected]) Summary Plant species that are native to Australia among many broad strategies and management options can be significant weeds of primary production and the available to achieve the aims of the Invasive Species environment, both within and outside their endemic Plan. New South Wales Department of Primary Indus- ranges. Of the 6049 taxa native to New South Wales, tries is the lead agency for weed management in the over 165 (2.7%) are considered to be weedy within state, and in partnership with Local Government enacts their indigenous range. With only one exception, none the Noxious Weeds Act 1993 (NWA). Having said this, are managed as noxious under the New South Wales there are a number of agencies, including New South Noxious Weeds Act 1993. Wales Department of Primary Industries (DPI), who Two groups of native species are managed as have responsibility for managing invasive plant spe- noxious weeds. With one exception, the first group cies impacting primary industries, the environment, includes a range of 14 tree, shrub, vine and epiphytic the community and the heritage/culture of all people, species found in northern and eastern rainforests and often using distinct legislation to help achieve this, high rainfall areas. These species are generally of and other objectives. concern outside their endemic range, for example The most recent review of the NWA identi- sweet pittosporum (Pittosporum undulatum Vent.), fied a number of conflicting interactions with other some lilly-pilly species (both Acmena and Syzygium New South Wales legislation (and attendant policies species), bower vine (Pandorea jasminoides (Lindl.) and procedures, Johnson and Lisle 2011). -

Darkwood Reserve NSW Report, 2010
Bush Blitz s pecies Discovery p r o g r a m Darkwood reserve NsW 12–16 February 2010 REPORT What is contents Bush Blitz? Bush Blitz is a three- What is Bush Blitz 2 year, multi-million dollar Executive summary 3 partnership between the Introduction 3 australian government, Reserve Overview 4 Bhp Billiton, earthwatch Methods 5 australia, and ausplots- Results 6 rangelands to document plants and animals in selected Discussion 7 properties across australia’s Appendix A: Species Lists 9 National reserve system. Fauna Taxa 10 Flora Taxa 16 Appendix B: Listed Species 19 this innovative partnership Fauna Taxa 20 harnesses the expertise of many Appendix C: Exotic Pest Species 21 of australia’s top scientists from Fauna Taxa 22 museums, herbaria, universities, Flora Taxa 22 and other institutions and organisations across the country. 2 Bush Blitz survey report Executive Introduction summary A short (six day) Bush Blitz was The Bush Blitz program aims to survey the flora and fauna of conducted on Darkwood Reserve in recent additions to the National Reserve System (NRS). Bush New South Wales during February 2010 Blitz is an initiative of the Australian Government, through the in conjunction with the Bush Blitz Australian Biological Resources Study (ABRS) in partnership with Media Launch. In total, 363 species were BHP Billiton, Earthwatch Australia and AusPlots-Rangelands. The identified on the reserve. With previous Bush Blitz objectives are: records for the Reserve, the total number ++ to promote, publicise and demonstrate the importance of of species known from Darkwood is taxonomy through the vehicle of species discovery; now 392. -
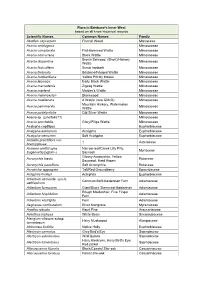
Flora in Brisbane's Inner West Based on All Know Historical Records
Flora in Brisbane's Inner West based on all know historical records Scientific Names Common Names Family Abutilon oxycarpum Flannel Weed Malvaceae Acacia amblygona Mimosaceae Acacia complanata Flat-stemmed Wattle Mimosaceae Acacia concurrens Black Wattle Mimosaceae Brown Salwood, (Short)/Hickory Acacia disparrima Mimosaceae Wattle Acacia fasiculifera Scrub Ironbark Mimosaceae Acacia fimbriata Brisbane/Fringed Wattle Mimosaceae Acacia hubbardiana Yellow Prickly Moses Mimosaceae Acacia leiocalyx Early Black Wattle Mimosaceae Acacia macradenia Zigzag Wattle Mimosaceae Acacia maidenii Maiden's Wattle Mimosaceae Acacia melanoxylon Blackwood Mimosaceae Acacia muellerana A Wattle (rare GMcD) Mimosaceae Mountain Hickory, Watermelon Acacia penninervis Mimosaceae Wattle Acacia podalyriifolia Qld Silver Wattle Mimosaceae Acacia sp. (juncifolia??) Mimosaceae Acacia spectabilis Glory/Piliga Wattle Mimosaceae Acalypha capillipes Euphorbiaceae Acalypha eremorum Acalypha Euphorbiaceae Acalypha nemorum Soft Acalypha Euphorbiaceae Acmella grandiflora var. ? Asteraceae brachyglossa Acmena smithii syns. Narrow-leaf/Creek Lilly Pilly, Myrtaceae Eugenia/Syzygium s. Satinash Glossy Acronychia, Yellow Acronychia laevis Rutaceae Boxwood, Hard Aspen Acronychia pauciflora Soft Acronychia Rutaceae Acrotriche aggregata Tall/Red Groundberry Epacridaceae Actephila lindleyi Actephila Euphorbiaceae Adiantum atroviride, syn A. Common/Soft Maidenhair Fern Adiantaceae aethiopicum Adiantum formosum Giant/Black Stemmed Maidenhair Adiantaceae Rough Maidenhair, Five Finger -

TAXON:Platycerium Superbum De Jonch. & Hennipman SCORE:4.0
TAXON: Platycerium superbum de SCORE: 4.0 RATING: Evaluate Jonch. & Hennipman Taxon: Platycerium superbum de Jonch. & Hennipman Family: Polypodiaceae Common Name(s): staghorn fern Synonym(s): Assessor: Chuck Chimera Status: Assessor Approved End Date: 11 Dec 2019 WRA Score: 4.0 Designation: EVALUATE Rating: Evaluate Keywords: Epiphytic Fern, Naturalized, Ornamental, Shade-Tolerant, Wind-Dispersed Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 203 Broad climate suitability (environmental versatility) y=1, n=0 n Native or naturalized in regions with tropical or 204 y=1, n=0 y subtropical climates Does the species have a history of repeated introductions 205 y=-2, ?=-1, n=0 y outside its natural range? 301 Naturalized beyond native range y = 1*multiplier (see Appendix 2), n= question 205 y 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see Appendix 2) y 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see Appendix 2) n 304 Environmental weed 305 Congeneric weed 401 Produces spines, thorns or burrs y=1, n=0 n 402 Allelopathic 403 Parasitic y=1, n=0 n 404 Unpalatable to grazing animals 405 Toxic to animals y=1, n=0 n 406 Host for recognized pests and pathogens 407 Causes allergies or is otherwise toxic to humans y=1, n=0 n 408 Creates a fire hazard in natural ecosystems y=1, n=0 n 409 Is a shade tolerant plant at some stage of its life cycle y=1, n=0 y Tolerates a wide range of soil conditions (or limestone 410 y=1, n=0 n conditions if not a volcanic island) Creation Date: 11 Dec 2019 (Platycerium superbum de Page 1 of 16 Jonch.