110 Exam5 Mccorkle SP16 Key
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
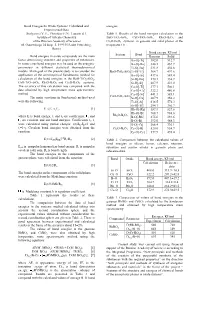
Bond Energies in Oxide Systems: Calculated and Experimental Data
Bond Energies In Oxide Systems: Calculated and energies. Experimental Data Stolyarova V. L., Plotnikov E.N., Lopatin S.I. Table 1. Results of the bond energies calculation in the Institute of Silicate Chemistry BaO-TiO 2-SiO 2, CaO-TiO 2-SiO 2, Rb 2O-B2O3 and of the Russian Academy of Sciences Cs 2O-B2O3 systems. in gaseous and solid phases at the ul. Odoevskogo 24 korp. 2, 199155 Sankt Petersburg, temperature 0 ¥ . Russia Bond energy, KJ/mol System Bond Bond energies in oxide compounds are the main Gaseous Solid factor determining structure and properties of substances. Ba-O[-Ti] 382.0 512.7 In some cases bond energies may be used as the energetic Ba-O[-Ba] 348.5 467.7 parameters in different statistical thermodynamical Ti-O[-Ba] 331.8 450.6 models. Main goal of the present study is to consider the BaO-TiO 2-SiO 2 Ti-O[-Ti] 364.7 497.6 application of the semiempirical Sanderson's method for Ba-O[-Si] 437.6 585.4 calculation of the bond energies in the BaO-TiO 2-SiO 2, Si-O[-Ba] 270.3 334.7 CaO-TiO 2-SiO 2, Rb 2O-B2O3 and Cs 2O-B2O3 systems. Si-O[-Si] 407.9 421.0 The accuracy of this calculation was compared with the Ca-O[-Ti] 377.1 500.1 data obtained by high temperature mass spectrometric Ca-O[-Ca] 322.2 486.8 method. Ca-O[-Si] 441.1 585.1 CaO-TiO -SiO The main equations in Sanderson's method used 2 2 Si-O[-Ca] 287.9 358.2 were the following. -

Molecular Orbital Theory to Predict Bond Order • to Apply Molecular Orbital Theory to the Diatomic Homonuclear Molecule from the Elements in the Second Period
Skills to Develop • To use molecular orbital theory to predict bond order • To apply Molecular Orbital Theory to the diatomic homonuclear molecule from the elements in the second period. None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. Previously, we described the electrons in isolated atoms as having certain spatial distributions, called orbitals, each with a particular orbital energy. Just as the positions and energies of electrons in atoms can be described in terms of atomic orbitals (AOs), the positions and energies of electrons in molecules can be described in terms of molecular orbitals (MOs) A particular spatial distribution of electrons in a molecule that is associated with a particular orbital energy.—a spatial distribution of electrons in a molecule that is associated with a particular orbital energy. As the name suggests, molecular orbitals are not localized on a single atom but extend over the entire molecule. Consequently, the molecular orbital approach, called molecular orbital theory is a delocalized approach to bonding. Although the molecular orbital theory is computationally demanding, the principles on which it is based are similar to those we used to determine electron configurations for atoms. -

Calculating Lattice Energies Using the Born-Haber Cycle an Extension Activity for AP Chemistry Students
Calculating Lattice Energies Using the Born-Haber Cycle An Extension Activity for AP Chemistry Students A particular set of equations known as a Born-Haber cycle demonstrates how chemists are able to use the first law of thermodynamics—that the energy of the universe is conserved in any chemical or physical change—to find an unknown energy value that is difficult or impossible to measure experimentally. Some energy quantities, such as the lattice energy of a mineral or the electron affinity of an atom, can be difficult to measure in the lab. Examining a Born-Haber cycle we see that there is more than one path to the formation of a substance in a particular state, and that if we use consistent definitions, an energy value that we seek can be calculated from energy values that we already know. The following exercise will help us see the way these energy values relate to one another, give us practice with their definitions and symbols, and deepen our insight to their meaning when we see them in other types of problems. Each physical or chemical change represented has: • an equation that represents a clearly defined physical or chemical change; • a definition of the particular type of energy change; • a symbol or abbreviation for the energy change equal to a value for the change in enthalpy (ΔH), the energy that is released or absorbed during the change, expressed in kJ/mol; and • a name by which that change in enthalpy is commonly known. To set up the equations in a Born-Haber cycle, cut out the cards for names, equations, defini- tions, and symbols with energy values. -

The Theoretical Study on Interaction of Hydrogen with Single-Walled Boron Nitride Nanotubes
THE JOURNAL OF CHEMICAL PHYSICS 123, 114703 ͑2005͒ The theoretical study on interaction of hydrogen with single-walled boron nitride nanotubes. I. The reactive force field ReaxFFHBN development ͒ Sang Soo Han, Jeung Ku Kang, and Hyuck Mo Leea Department of Materials Science and Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 305-701, Republic of Korea Adri C. T. van Duin and William A. Goddard III Materials and Process Simulation Center, California Institute of Technology, California 91125 ͑Received 2 November 2004; accepted 21 June 2005; published online 19 September 2005͒ We present a new reactive force field ReaxFFHBN derived to accurately model large molecular and condensed phase systems of H, B, and N atoms. ReaxFFHBN has been tested against quantum calculation data for B–H, B–B, and B–N bond dissociations and for H–B–H, B–N–B, and N–B–N bond angle strain energies of various molecular clusters. The accuracy of the developed ReaxFFHBN for B–N–H systems is also tested for ͑i͒ H–B and H–B bond energies as a function of out of plane ͑ ͒ ͑ ͒ ͑ ͒ in H–B NH2 3 and H–N BH2 3, respectively, ii the reaction energy for the B3N3H6 +H2 → ͑ ͒ B3N3H8, and iii crystal properties such as lattice parameters and equations of states for the hexagonal type ͑h-BN͒ with a graphite structure and for the cubic type ͑c-BN͒ with a zinc-blende structure. For all these systems, ReaxFFHBN gives reliable results consistent with those from quantum calculations as it describes well bond breaking and formation in chemical processes and physical properties. -

Session #9: Homework Solutions
Session #9: Homework Solutions Problem #1 Estimate the ionic radius of Cs+. The lattice energy of CsCl is 633 kJ/mol. For CsCl the Madelung constant, M, is 1.763, and the Born exponent, n, is 10.7. The ionic radius of Cl– is known to be 1.81 Å. Solution Mq q ⎛⎞1 E1 = 1 2 −= and r r+r lattice ⎜⎟ o Cs+ Cl− 4rπεoo⎝⎠ n Solve first for ro MqqN ⎛⎞1 1.763(1.6 x 10-19 ) 2 6.02 x 1023 ⎛ 1 ⎞ r1 = 12 Aν −− = 1 o ⎜⎟ -12 5 ⎜ ⎟ 4Eπεolattice⎝⎠ n 4π 8.85 x 10 6.33x10 ⎝10.7⎠ = 3.50 x 10-10 m = 3.50 Å = r+ r Cs+- Cl ∴ r = 3.50-1.81 = 1.69 Å Cs+ Problem #2 (a) CFCs have been implicated in ozone depletion. Show that when Freon 12 (CCl2F2) is exposed to ultraviolet radiation, the compound decomposes to produce chlorine. (b) Draw the Lewis structure of Freon 12 and indicate the polarities of each bond within this compound. (c) Determine the percent ionic character of the C--Cl and C--F bonds. DATA: Average Bond Energies (kJ/mol) Single Bonds Multiple Bonds H-H 435 C = C 610 F-F 155 C ≡ C 836 Cl-Cl 242 C-C 347 Solution (a) Chlorine will be liberated if the C-Cl bond breaks, therefore, compute its strength and show that ultraviolet photons have enough energy to break the bond. 2 ECCl−−− =+ E CCx9 E ClCl 6.3(χχC− Cl) = 347 x 242 + 96.3(2.55 - 3.16)2 = 326 x 103/6.02 x 1023 = 5.41 x 10-19 J/bond photon will break ∴this bond if Eph > Ebond ⇒ critical λ is hc 6.6 x 10-34 x 3 x 108 λ = = = 3.66 x 10-7 m -19 Ebond 5.41 x 10 which lies in the u.v. -

Real Space in Situ Bond Energies: Toward a Consistent
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repositorio Institucional de la Universidad de Oviedo Real space in situ bond energies: toward a consistent energetic definition of bond strength D. Menéndez-Crespo, A. Costales, E. Francisco, and A. Martín Pendása∗ June 20, 2018 Abstract A rigorous definition of intrinsic bond strength based on the partitioning of a molecule into real space fragments is presented. Using the domains provided by the quantum theory of atoms in molecules (QTAIM) together with the interacting quantum atoms (IQA) energetic decomposition, we show how an in situ bond strength, matching all the requirements of an intrinsic bond energy, can be defined between each pair of fragments. Total atomization or fragmentation energies are shown to be equal to the sum of these in situ bond energies (ISBEs) if the energies of the fragments are measured with respect to their in-the-molecule state. These energies usually lie above the ground state of the isolated fragments by quantities identified with the standard frag- ment relaxation or deformation energies, which are also provided by the protocol. Deformation energies bridge dissociation energies with ISBEs, and can be dissected using well-known tools of real space theories of chemical bonding. Similarly, ISBEs can be partitioned into ionic and covalent contributions, and this feature adds to the chemical appeal of the procedure. All the energetic quantities examined are observable and amenable, in principle, to experimental deter- mination. Several systems, exemplifying the role of each energetic term herein presented are used to show the power of the approach. -

Valence Bond Theory
Valence Bond Theory • A bond is a result of overlapping atomic orbitals from two atoms. The overlap holds a pair of electrons. • Normally each atomic orbital is bringing one electron to this bond. But in a “coordinate covalent bond”, both electrons come from the same atom • In this model, we are not creating a new orbital by the overlap. We are simply referring to the overlap between atomic orbitals (which may or may not be hybrid) from two atoms as a “bond”. Valence Bond Theory Sigma (σ) Bond • Skeletal bonds are called “sigma” bonds. • Sigma bonds are formed by orbitals approaching and overlapping each other head-on . Two hybrid orbitals, or a hybrid orbital and an s-orbital, or two s- orbitals • The resulting bond is like an elongated egg, and has cylindrical symmetry. Acts like an axle • That means the bond shows no resistance to rotation around a line that lies along its length. Pi (π) Bond Valence Bond Theory • The “leftover” p-orbitals that are not used in forming hybrid orbitals are used in making the “extra” bonds we saw in Lewis structures. The 2 nd bond in a double bond nd rd The 2 and 3 bonds in a triple bond /~harding/IGOC/P/pi_bond.html • Those extra bonds form only after the atoms are brought together by the formation of the skeletal bonds made by www.chem.ucla.edu hybrid orbitals. • The “extra” π bonds are always associated with a skeletal bond around which they form. • They don’t form without a skeletal bond to bring the p- orbitals together and “support” them. -

Chapter 5 the Covalent Bond 1
Chapter 5 The Covalent Bond 1. What two opposing forces dictate the bond length? (Why do bonds form and what keeps the bonds from getting any shorter?) The bond length is the distance at which the repulsion of the two nuclei equals the attraction of the valence electrons on one atom and the nucleus of the other. 3. List the following bonds in order of increasing bond length: H-Cl, H-Br, H-O. H-O < H-Cl < H-Br. The order of increasing atom size. 5. Use electronegativities to describe the nature (purely covalent, mostly covalent, polar covalent, or ionic) of the following bonds. a) P-Cl Δχ = 3.0 – 2.1 = 0.9 polar covalent b) K-O Δχ = 3.5 – 0.8 = 2.7 ionic c) N-H Δχ = 3.0 – 2.1 = 0.9 polar covalent d) Tl-Br Δχ = 2.8 – 1.7 = 1.1 polar covalent 7. Name the following compounds: a) S2Cl2 disulfur dichloride b) CCl4 carbon tetrachloride c) PCl5 phosphorus pentachloride d) HCl hydrogen chloride 9. Write formulas for each of the following compounds: a) dinitrogen tetroxide N2O4 b) nitrogen monoxide NO c) dinitrogen pentoxide N2O5 11. Consider the U-V, W-X, and Y-Z bonds. The valence orbital diagrams for the orbitals involved in the bonds are shown in the margin. Use an arrow to show the direction of the bond dipole in the polar bonds or indicate “not polar” for the nonpolar bond(s). Rank the bonds in order of increasing polarity. The bond dipole points toward the more electronegative atom in the bond, which is the atom with the lower energy orbital. -

Carbon Carbon Spsp Hybrids : Acetylene and the : Acetylene And
CarbonCarbon spsp hybridshybrids:: AcetyleneAcetylene andand thethe TripleTriple bondbond CC2HH2 isis HH--CCCC--HH FormForm spsp onon eacheach CC leavingleaving 2p2px,, 2p2py “unused”“unused” H - sp C sp + +-+ sp C sp H PredictsPredicts linearlinear structure.structure. AboveAbove areare allall σσ bondsbonds H-C-C-H Uses up 2 valence e- for each C in sp (and 2 from 1s H). Leaves 2 valence e - for each C unused. 2py 2py Put last valence 2px e- into ππ orbitals sp formed from H C C H 2px, 2py 2px - - 2 e in πx 2 e in πy Gives two π bonds connecting carbon atoms. π bonds are at right angles to each other. Total C-C bonds are now 3, one σσ, 2 π Short Comparison of Bond Order, Bond Length, Bond Energy C-C C-C C-C Molecule Bond Order Bond Length Bond E, kcal/mole σσ Ethane, C2H6 1 (1 ) 1.54Å 83 σσ Ethylene, C2H4 2 (1 , 1π) 1.35Å 125 σσ Acetylene, C2H2 3 (1 , 2π) 1.21Å 230 ConjugationConjugation andand DelocalizationDelocalization ofof ElectronsElectrons andand BondsBonds Although energy of π* in ethylene < σ*, conjugated polylenes have even lower energy π* levels. These absorb light at longer wavelength- sometimes even in visible (human eye’s light perception). Conjugated polyenes: C=C-C-C=C-C 2 essentially independent double bonds. C=C-C=C-C=C polyene DoubleDouble bondsbonds ALTERNATE!ALTERNATE! Could draw ππ bonds between any 2 C’s ππ* bonds C C C C C C drawn are not unique! ψψ This gives delocalized structure MO = (Const)[2py(1) + 2py(2) + 2py(3) + 2py(4) +2py(5) + 2py(6) +………]. -

Bond Orders of the Diatomic Molecules†
RSC Advances PAPER View Article Online View Journal | View Issue Bond orders of the diatomic molecules† Cite this: RSC Adv.,2019,9,17072 Taoyi Chen and Thomas A. Manz * Bond order quantifies the number of electrons dressed-exchanged between two atoms in a material and is important for understanding many chemical properties. Diatomic molecules are the smallest molecules possessing chemical bonds and play key roles in atmospheric chemistry, biochemistry, lab chemistry, and chemical manufacturing. Here we quantum-mechanically calculate bond orders for 288 diatomic molecules and ions. For homodiatomics, we show bond orders correlate to bond energies for elements within the same chemical group. We quantify and discuss how semicore electrons weaken bond orders for elements having diffuse semicore electrons. Lots of chemistry is effected by this. We introduce a first-principles method to represent orbital-independent bond order as a sum of orbital-dependent bond order components. This bond order component analysis (BOCA) applies to any spin-orbitals that are unitary transformations of the natural spin-orbitals, with or without periodic boundary conditions, and to non-magnetic and (collinear or non-collinear) magnetic materials. We use this BOCA to study all À Creative Commons Attribution-NonCommercial 3.0 Unported Licence. period 2 homodiatomics plus Mo2,Cr2, ClO, ClO , and Mo2(acetate)4. Using Manz's bond order equation with DDEC6 partitioning, the Mo–Mo bond order was 4.12 in Mo2 and 1.46 in Mo2(acetate)4 with a sum of bond orders for each Mo atom of 4. Our study informs both chemistry research and education. As Received 5th February 2019 a learning aid, we introduce an analogy between bond orders in materials and message transmission in Accepted 10th May 2019 computer networks. -

Influence of Aromaticity on Excited State Structure, Reactivity and Properties
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1679 Influence of Aromaticity on Excited State Structure, Reactivity and Properties KJELL JORNER ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 ISBN 978-91-513-0354-3 UPPSALA urn:nbn:se:uu:diva-349229 2018 Dissertation presented at Uppsala University to be publicly examined in room 80101, Ångströmlaboratoriet, Lägerhyddsvägen 1, Uppsala, Thursday, 14 June 2018 at 13:15 for the degree of Doctor of Philosophy. The examination will be conducted in English. Faculty examiner: Prof. Dr. Rainer Herges (Christian-Albrechts-Universität zu Kiel, Otto-Diels- Institut für Organische Chemie). Abstract Jorner, K. 2018. Influence of Aromaticity on Excited State Structure, Reactivity and Properties. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1679. 55 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-513-0354-3. This thesis describes work that could help development of new photochemical reactions and light-absorbing materials. Focus is on the chemical concept "aromaticity" which is a proven conceptual tool in developing thermal chemical reactions. It is here shown that aromaticity is also valuable for photochemistry. The influence of aromaticity is discussed in terms of structure, reactivity and properties. With regard to structure, it is found that photoexcited molecules change their structure to attain aromatic stabilization (planarize, allow through-space conjugation) or avoid antiaromatic destabilization (pucker). As for reactivity, it is found that stabilization/destabilization of reactants decrease/increase photoreactivity, in accordance with the Bell-Evans-Polanyi relationship. Two photoreactions based on excited state antiaromatic destabilization of the substrates are reported. -

The Nature of the Chemical Bond. Iv. the Energy of Single Bonds and the Relative Electronegativity of Atoms
3570 [CONTRIBUTION FROM THE GATES CHEMICAL LABORATORY, CALIFORNIA INSTITUE OF TECHNOLOGY, NO. 326] THE NATURE OF THE CHEMICAL BOND. IV. THE ENERGY OF SINGLE BONDS AND THE RELATIVE ELECTRONEGATIVITY OF ATOMS BY LINUS PAULING Journal of the American Chemical Society Volume 54, p. 3570-3582 September 1932 RECEIVED MAY 18, 1932 PUBLISHED SEPTEMBER 5, 1932 Recent developments in the application of the quantum mechanics to problems of molecular structure1 have indicated that the properties of a bond between two atoms often are determined mainly by one single-electron orbital wave function for each atom, and are not strongly affected by the other atoms in the molecule; for when the wave function for a molecule containing single covalent bonds is set up with the use of single-electron orbital wave functions, it is found that each bond function tends to overlap to the maximum extent the other function involved in one bond, and to avoid overlapping all others, so that the resonance integrals other than those characteristics of the bonds are small. The empirical evidence of interatomic distances supports this view; it has been possible to formulate a set of covalent radii for use in purely covalent molecules (in which each atom forms covalent bonds to a number determined by its position in the periodic system) which accounts satisfactory for observed distance in molecules to which a single Lewis electronic structure can be assigned.2 It has also been found that changing the covalence of an atom, and hence the nature of the bond function, produces a change in the radius.3 Independence of the bonds in a molecule would require that the total energy of formation of the molecule from separate atoms be expressible as the sum of constant energy terms characteristic of the various bonds.