Cunene Labeo (Labeo Ansorgii) Ecological Risk Screening Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Instituto Da Cooperação Portuguesa (Portugal)
Instituto da Cooperação Portuguesa (Portugal) Ministério da Energia e Águas de Angola SOUTHERN AFRICAN DEVELOPMENT COMMUNITY PLAN FOR THE INTEGRATED UTILIZATION OF THE WATER RESOURCES OF THE HYDROGRAPHIC BASIN OF THE CUNENE RIVER SYNTHESIS LNEC – Laboratório Nacional de Engenharia Civil Page 1/214 LNEC – Proc.605/1/11926 MINISTÉRIO DO EQUIPAMENTO SOCIAL Laboratório Nacional de Engenharia Civil DEPARTMENT OF HYDRAULICS Section for Structural Hydraulics Proc.605/1/11926 PLAN FOR THE INTEGRATED UTILIZATION OF THE WATER RESOURCES OF THE HYDROGRAPHIC BASIN OF THE CUNENE RIVER Report 202/01 – NHE Lisbon, July 2001 A study commissioned by the Portuguese Institute for Cooperation I&D HYDRAULICS Page 2/214 LNEC – Proc.605/1/11926 PLAN FOR THE INTEGRATED UTILIZATION OF THE WATER RESOURCES OF THE HYDROGRAPHIC BASIN OF THE CUNENE RIVER SYNTHESIS Page 3/214 LNEC – Proc.605/1/11926 PLAN FOR THE INTEGRATED UTILIZATION OF THE WATER RESOURCES OF THE HYDROGRAPHIC BASIN OF THE CUNENE RIVER INTRODUCTORY NOTE This report synthesizes a number of documents that have been elaborated for the Portuguese Institute for Cooperation. The main objective of the work was to establish a Plan for the Integrated Utilization of the Water Resources of the Hydrographic Basin of the Cunene River. As the elaboration of this Plan is a multi-disciplinary task, it was deemed preferable to grant independence of reporting on the work of each team that contributed to the final objective. That is why each report consists of a compilation of volumes. REPORT I VOLUME 1 – SYNTHESIS (discarded) -

Angolan Giraffe (Giraffa Camelopardalis Ssp
Angolan Giraffe (Giraffa camelopardalis ssp. angolensis) Appendix 1: Historical and recent geographic range and population of Angolan Giraffe G. c. angolensis Geographic Range ANGOLA Historical range in Angola Giraffe formerly occurred in the mopane and acacia savannas of southern Angola (East 1999). According to Crawford-Cabral and Verissimo (2005), the historic distribution of the species presented a discontinuous range with two, reputedly separated, populations. The western-most population extended from the upper course of the Curoca River through Otchinjau to the banks of the Kunene (synonymous Cunene) River, and through Cuamato and the Mupa area further north (Crawford-Cabral and Verissimo 2005, Dagg 1962). The intention of protecting this western population of G. c. angolensis, led to the proclamation of Mupa National Park (Crawford-Cabral and Verissimo 2005, P. Vaz Pinto pers. comm.). The eastern population occurred between the Cuito and Cuando Rivers, with larger numbers of records from the southeast corner of the former Mucusso Game Reserve (Crawford-Cabral and Verissimo 2005, Dagg 1962). By the late 1990s Giraffe were assumed to be extinct in Angola (East 1999). According to Kuedikuenda and Xavier (2009), a small population of Angolan Giraffe may still occur in Mupa National Park; however, no census data exist to substantiate this claim. As the Park was ravaged by poachers and refugees, it was generally accepted that Giraffe were locally extinct until recent re-introductions into southern Angola from Namibia (Kissama Foundation 2015, East 1999, P. Vaz Pinto pers. comm.). BOTSWANA Current range in Botswana Recent genetic analyses have revealed that the population of Giraffe in the Central Kalahari and Khutse Game Reserves in central Botswana is from the subspecies G. -

The Mayflies (Ephemeroptera) of Angola—New Species and Distribution Records from Previously Unchartered Waters, with a Provisional Species Checklist
Zoosymposia 16: 124–138 (2019) ISSN 1178-9905 (print edition) http://www.mapress.com/j/zs/ ZOOSYMPOSIA Copyright © 2019 · Magnolia Press ISSN 1178-9913 (online edition) http://dx.doi.org/10.11646/zoosymposia.16.1.11 http://zoobank.org/urn:lsid:zoobank.org:pub:EEC14B5A-21DE-4FB7-8D62-AD29C65F35BA The Mayflies (Ephemeroptera) of Angola—new species and distribution records from previously unchartered waters, with a provisional species checklist HELEN M. BARBER-JAMES1,2,3*& INA S. FERREIRA2,1,3 1 Department of Freshwater Invertebrates, Albany Museum, Somerset Street, Makhanda (Grahamstown) 6139, South Africa. 2 Department of Zoology and Entomology, Rhodes University, Makhanda (Grahamstown) 6140, South Africa. 3 National Geographic Okavango Wilderness Project, Wild Bird Trust, South Africa. (*) Corresponding author: [email protected] Abstract A preliminary assessment of Ephemeroptera species diversity in Angolan freshwater ecosystems is presented. The results are based on three surveys carried out between 2016 and 2018, supplemented by literature synthesis. The area studied includes headwater streams and tributaries feeding the Okavango Delta, namely the Cubango, Cuito and Cuanavale Rivers, which together produced 35 species, and those flowing into the Zambezi River system, the Cuembo, Cuando, Luanginga and Lungué-Bungo Rivers which together produced 29 species. Twenty-one species were identified from the Cubango River, a fast flowing, rocky substrate river, different in character to all the other rivers surveyed. The other rivers, which all flow over Kalahari sand substrate with dense rooted aquatic macrophytes, generally lacking rocky substrate, produced 33 species between them. Prior to this research, only one mayfly species had been described from Angola in 1959. -

Namibia and Angola: Analysis of a Symbiotic Relationship Hidipo Hamutenya*
Namibia and Angola: Analysis of a symbiotic relationship Hidipo Hamutenya* Introduction Namibia and Angola have much in common, but, at the same time, they differ greatly. For example, both countries fought colonial oppression and are now independent; however, one went through civil war, while the other had no such experience. Other similarities include the fact that the former military groups (Angola’s Movimiento Popular para la Liberacão de Angola, or MPLA, and Namibia’s South West Africa People’s Organisation, or SWAPO) are now in power in both countries. At one time, the two political movements shared a common ideological platform and lent each other support during their respective liberation struggles. The two countries are also neighbours, with a 1,376-km common border that extends from the Atlantic Ocean in the east to the Zambezi River in the west. Families and communities on both sides of the international boundary share resources, communicate, trade and engage in other types of exchange. All these facts point to a relationship between the two countries that goes back many decades, and continues strongly today. What defines this relationship and what are the crucial elements that keep it going? Angola lies on the Atlantic coast of south-western Africa. It is richly endowed with natural resources and measures approximately 1,246,700 km2 in land surface area. Populated with more than 14 million people, Angola was a former Portuguese colony. Portuguese explorers first came to Angola in 1483. Their conquest and exploitation became concrete when Paulo Dias de Novais erected a colonial settlement in Luanda in 1575. -

The Kunene River Mouth: Managing a Unique Environment
THE KUNENE RIVER MOUTH: MANAGING A UNIQUE ENVIRONMENT by JOHN RICHARD BERNARD PATERSON Submitted in partial fulfillment of the requirements for the degree of Master of Environment and Development in the Centre for Environment, Agriculture and Development, School of Applied Environmental Sciences University of KwaZulu-Natal Pietermaritzburg 2007 Abstract The Kunene River Mouth (KRM) is one of only two river mouths in Namibia. The Kunene river and river mouth is bisected by the international border between Namibia and Angola, and lies between two protected areas, Iona National Park in Angola and Skeleton Coast Park in Namibia. The governments of Namibia and Angola have signed a Memorandum of Understanding (MoU) to link these two parks as a transfrontier park. This study further proposes a transfrontier Marine Protected Area to protect the marine environment surrounding the KRM and the Angola Benguela Front. The KRM is a fluvially dominated freshwater river mouth. The area is a biogeographically important biodiversity hotspot. The remoteness and pristine character contribute to the aesthetic appeal of the area. This study provides a profile of the KRM addressing its conservation value in terms of both biodiversity and aesthetic value, making use of the concept of “sense of place”. An analysis of all current and potential stakeholders is presented and their interests, activities and potential threats are evaluated. The main stakeholders are Government: the Ministry of Environment and Tourism, the Angolan Government, Ministry of Fisheries and Marine Resources, Namwater, Ministry of Mines and Energy, and the Kunene Regional Council. The private sector presently has a small stake in the area, with the exception of the Northern Namibia Development Corporation who is prospecting for diamonds at the KRM. -
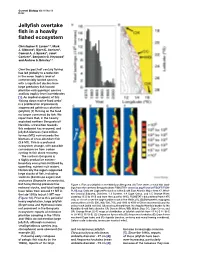
Jellyfish Overtake Fish in a Heavily Fished Ecosystem
Current Biology Vol 16 No 13 R492 Jellyfish overtake fish in a heavily fished ecosystem Christopher P. Lynam1,6, Mark J. Gibbons2, Bjørn E. Axelsen3, Conrad A. J. Sparks4, Janet Coetzee5, Benjamin G. Heywood1 and Andrew S. Brierley1,7 Over the past half century fishing has led globally to a reduction in the mean trophic level of commercially landed species, with a significant decline from large predatory fish toward plankton-eating pelagic species and low trophic-level invertebrates [1]. An implied endpoint of this ‘fishing down marine food webs’ is a proliferation of previously suppressed gelatinous plankton (jellyfish) [2] thriving on the food no longer consumed by fish. We report here that, in the heavily exploited northern Benguela off Namibia, a transition towards this endpoint has occurred, and jellyfish biomass (12.2 million tonnes (MT)) now exceeds the biomass of once-abundant fish (3.6 MT). This is a profound ecosystem change, with possible consequences from carbon cycling to fish stock recovery. The northern Benguela is a highly productive eastern- boundary ecosystem fertilised by upwelling, nutrient-rich waters. Historically the region supported large stocks of fish, including sardines (Sardinops sagax) and anchovies (Engraulis encrasicolis), but heavy fishing pressure has Figure 1. Fish and jellyfish in the Namibian Benguela. (A) Time series of total fish land- reduced stocks, and total landings ings from the northern Benguela (from FISHSTAT+ www.fao.org/fi/statist/FISOFT/FISH- have fallen from around 17 MT in PLUS.asp. Data are Capture Production in the South East Atlantic Major Area 47, West- the late 1970s to just 1 MT now ern Coastal Subarea, Divisions 1.3 Cunene, 1.4 Cape Cross, and 1.5 Orange River, (Figure 1A). -

River Keepers-Forpdf-4
RIVER KEEPERS HANDBOOK A Guide to Protecting Rivers and Catchments in Southern Africa International Rivers Network RIVER KEEPERS HANDBOOK A Guide to Protecting Rivers and Catchments in Southern Africa by Lori Pottinger Published by International Rivers Network Editorial Team Tendai Chitewere L Liane Greeff L Ryan Hoover Steve Rothert L Olive Sephuma L Richard Sherman Acknowledgements Many people contributed to this booklet, adding insights, information and ideas on how to make it a more useful document. In particular, we'd like to thank Keith Cooper, Richard Hunt, Chris Neme, Mike Scholand, Bahman Sheikh, the Desert Research Foundation of Namibia and everyone at International Rivers Network. Special thanks to the Richard and Rhoda Goldman Fund and the Compton Foundation for making this booklet possible. Published by International Rivers Network, Berkeley, CA, USA, 1999 ISBN 0-9662771-2-0 Designed by Jeanette Madden Graphic Design Printed by West Coast Print Center TABLE OF CONTENTS Introduction . 1 Part 1 Catchment Basics What is a Catchment? . 3 Sidebars and Graphics Water in the Landscape . 4 The Water Cycle . 5 Key Issues in Water Management . 6 Part 2 Threats to Catchments . 8 The Big Dams Debate . 13 Sidebars and Graphics Southern African Water Index. 11 Sustainable Water Planning . 13 The Major Impacts of Dams . 15 The World Commission on Dams . 18 Map: Rivers in Peril . 19 River Projects to Watch. 20 Part 3 Making a Difference Becoming a Catchment Keeper . 22 Hope for the Future: Creating Catchment Communities. 27 New Approaches to Energy and Water Supply. 31 Sidebars and Graphics Rethinking the Planning Process. 24 Write Letters! . -

S Angola on Their Way South
Important Bird Areas in Africa and associated islands – Angola ■ ANGOLA W. R. J. DEAN Dickinson’s Kestrel Falco dickinsoni. (ILLUSTRATION: PETE LEONARD) GENERAL INTRODUCTION December to March. A short dry period during the rains, in January or February, occurs in the north-west. The People’s Republic of Angola has a land-surface area of The cold, upwelling Benguela current system influences the 1,246,700 km², and is bounded by the Atlantic Ocean to the west, climate along the south-western coast, and this region is arid in the Republic of Congo to the north-west, the Democratic Republic of south to semi-arid in the north (at about Benguela). Mean annual Congo (the former Zaïre) to the north, north-east, and east, Zambia temperatures in the region, and on the plateau above 1,600 m, are to the south-east, and Namibia to the south. It is divided into 18 below 19°C. Areas with mean annual temperatures exceeding 25°C (formerly 16) administrative provinces, including the Cabinda occur on the inner margin of the Coast Belt north of the Queve enclave (formerly known as Portuguese Congo) that is separated river and in the Congo Basin (Huntley 1974a). The hottest months from the remainder of the country by a narrow strip of the on the coast are March and April, during the period of heaviest Democratic Republic of Congo and the Congo river. rains, but the hottest months in the interior, September and October, The population density is low, c.8.5 people/km², with a total precede the heaviest rains (Huntley 1974a). -

An Aerial Photographic Wildlife Survey of the Iona National Park, Angola November 2016 to February 2017
An aerial photographic wildlife survey of the Iona National Park, Angola November 2016 to February 2017 Table of contents 1. Introduction ................................................................................................................................... 2 1.1 Study rationale ........................................................................................................................ 2 1.2 Study area ................................................................................................................................ 4 2. Methodology .................................................................................................................................. 7 2.1 Aerial photography ................................................................................................................. 7 2.2 Geo-referencing of wildlife sightings ..................................................................................... 8 2.3 Identification of wildlife species ............................................................................................. 9 2.4 Determination of wildlife estimates ...................................................................................... 10 2.5 Spatial analysis ...................................................................................................................... 10 2.5.1 Species distribution and density heat-maps .................................................................. 10 2.5.2 Graze and browse biomass estimates for terrestrial -

The Okavango River Basin in Southern Africa: a Case Study of Transboundary Resource Management Issues
University of Colorado Law School Colorado Law Scholarly Commons Allocating and Managing Water for a Sustainable Future: Lessons from Around the 2002 World (Summer Conference, June 11-14) 6-14-2002 The Okavango River Basin in Southern Africa: A Case Study of Transboundary Resource Management Issues Robert K. Hitchcock Follow this and additional works at: https://scholar.law.colorado.edu/allocating-and-managing-water-for- sustainable-future Part of the Environmental Policy Commons, Natural Resources and Conservation Commons, Natural Resources Management and Policy Commons, Sustainability Commons, Transnational Law Commons, Water Law Commons, and the Water Resource Management Commons Citation Information Hitchcock, Robert K., "The Okavango River Basin in Southern Africa: A Case Study of Transboundary Resource Management Issues" (2002). Allocating and Managing Water for a Sustainable Future: Lessons from Around the World (Summer Conference, June 11-14). https://scholar.law.colorado.edu/allocating-and-managing-water-for-sustainable-future/71 Reproduced with permission of the Getches-Wilkinson Center for Natural Resources, Energy, and the Environment (formerly the Natural Resources Law Center) at the University of Colorado Law School. Robert K. Hitchcock, The Okavango River Basin in Southern Africa: A Case Study of Transboundary Resource Management Issues, in ALLOCATING AND MANAGING WATER FOR A SUSTAINABLE FUTURE: LESSONS FROM AROUND THE WORLD (Natural Res. Law Ctr., Univ. of Colo. Sch. of Law 2002). Reproduced with permission of the Getches-Wilkinson Center for Natural Resources, Energy, and the Environment (formerly the Natural Resources Law Center) at the University of Colorado Law School. The Okavango River Basin in Southern Africa: A Case Study of Transboundary Resource Management Issues Robert K. -

Country: South Africa, Namibia
Country: Angola Research vessel: R/V DR. FRIDTJOF NANSEN Survey number: 2012403 Number of days: 27 General objectives: Survey of the fish resources of Angola Port Date Coverage Specific objectives Departure Luanda 2 April Angola • To survey, map and describe the distribution, composition and abundance of the main demersal species, with special emphasis on sea breams (Sparidae), croakers (Sciaenidae), grunts (Haemulidae), groupers (Serranidae), hakes (Merlucciidae), cephalopods and shrimps (Parapenaeus longirostris and Aristeus varidens) on the Angolan shelf and slope (down to 800 m), from Cunene River (17°14’S) to Tombua(15°40’S), and from Benguela (12°35’S) to Congo River (06°00’S) using bottom trawl and the swept-area method. • To collect biological data such as length, weight, sex and maturity stage of Dentex macrophthalmus, D. angolensis, Pagellus Arrival Luanda 29 April bellottii, Umbrina canariensis, Merluccius polli, M. capensis, M. paradoxus, Brachydeuterus auritus, A. varidens, Parapenaeus longirostris and Chaceon maritae. • To collect the stomach contents for some species such as U. canariensis and B.auritus and Merluccius polli for subsequent analyses in the INIP Lab. • To monitor the general hydrographical conditions using CTD-sonde on each trawlstation and map the temperature, salinity and oxygen. • To carry out a monitoring line at Cunene River using INIP’s new standard hydrographical profiles for collection of temperature, salinity and oxygen, water nutrients and phytoplankton. Cruise leader: Diana Zaera Participants: From INIP, Angola: 01.04-23.04: Silvi Nsiangango (local cruise leader), Antonio Buco, Fátima Delicado, Domingos Pedro, María Margarida, Nilsa Alves, Eridson Sequenha, Noémia Nganga, Enoque Canganjo, Bernardo Fernandes. -

Placer Formation and Termination of the Orange Sand Hi
Page 1 of 81 Sedimentology 1 1 2 3 4 ������������ ���������� ������������ ���������� ������ ��� ��������� 5 6 ���������� �� ������� ���������� ���� ������������ ��� ���� ������� 7 8 �������������������������������� 9 10 11 EDUARDO GARZANTI1*, PEDRO DINIS2, PIETER VERMEESCH3, SERGIO ANDÒ1, 12 13 ANNETTE HAHN4, JOÃO HUVI5, MARA LIMONTA1, MARTA PADOAN1 ALBERTO 14 1 3 1 15 RESENTINI , MARTIN RITTNER , GIOVANNI VEZZOLI 16 17 18 19 �� 20 ����������� ���� ����������� ��������� ����������� ��� ������ ���� ������������������������ ����������� ��� 21 ������������������������������������ 22 2� ��������� ������� ��� ���� �� ���������� ������������� ��� ��������� ��� ������� ������������� ��� 23 ������������������������������������������������������������� 24 3 25 � ������� �������������� �������� ����������� ��� ������ ��������� ����������� �������� �������� ������� 26 ������������ 27 4�������������������������������������������������������������������������������������� 28 5 29 ���������������������������������������������������������������������������� � 30 31 ��Corresponding author. Tel.: +39-02-64482088 32 33 34 E-mail: [email protected] (E. Garzanti), [email protected] (P. Dinis), [email protected] 35 (P.Vermeesch), [email protected] (S. Andò), [email protected] (A.Hahn), 36 [email protected] (J.Huvi), [email protected] (M.Limonta), [email protected] 37 38 (M.Padoan), [email protected] (A. Resentini), [email protected] (M.Rittner), 39 [email protected] (G. Vezzoli) 40 � 41 � 42 43 � 44