Lepidoptera: Sesiidae) Using Cytochrome Oxidase I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lilac (Ash) Borer Pupal Skins Extrude from Trunk
Lilac (Ash) Borer Pupal skins extrude from trunk Name and Description—Podosesia syringae (Harris) [Lepidoptera: Sesiidae] Adult lilac (ash) borers are moths that vary in color from brown to yel- low to orange. They have clear wings with a span of about 1-1 1/8 inches (26-28 mm) and appear wasp-like (fig. 1). Larvae are about 1 inch (2.5 cm) long and are white with brown heads (fig. 2). Hosts—Ash and lilac Life Cycle—There is one generation per year. Mature borer larvae over- winter in tunnels under the bark. Adult moths emerge from March through June to lay eggs on the bark of host trees. The larvae bore into trunks and branches of the sapwood of trees during the summer. Galleries can be up to 6 inches (15 cm) long. Figure 1. Adult ash borer. Photo: Daniel Herms, Ohio State University, Bugwood.org. Damage—The mining of the larvae causes branch dieback (fig. 3). It can also lead to broken branches. The leaves on affected branches turn brown as the branch dies. Extensive mining can also lead to tree death. Entrances to lar- val mines often appear as sunken or cankered areas on the bark of the trunk or branch. Dark, moist sawdust can be found around the Figure 2. Ash borer larva. Photo: James Solomon, USDA Forest gallery entrance (fig. 4). Pupal Service, Bugwood.org. skin remaining in the bark is often also observed (fig. 5). Management—Avoid damaging trees—maintaining trees in good health reduces their susceptibil- ity to attack. There are chemical sprays that are highly effective at Figure 3. -

Diseases of Trees in the Great Plains
United States Department of Agriculture Diseases of Trees in the Great Plains Forest Rocky Mountain General Technical Service Research Station Report RMRS-GTR-335 November 2016 Bergdahl, Aaron D.; Hill, Alison, tech. coords. 2016. Diseases of trees in the Great Plains. Gen. Tech. Rep. RMRS-GTR-335. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 229 p. Abstract Hosts, distribution, symptoms and signs, disease cycle, and management strategies are described for 84 hardwood and 32 conifer diseases in 56 chapters. Color illustrations are provided to aid in accurate diagnosis. A glossary of technical terms and indexes to hosts and pathogens also are included. Keywords: Tree diseases, forest pathology, Great Plains, forest and tree health, windbreaks. Cover photos by: James A. Walla (top left), Laurie J. Stepanek (top right), David Leatherman (middle left), Aaron D. Bergdahl (middle right), James T. Blodgett (bottom left) and Laurie J. Stepanek (bottom right). To learn more about RMRS publications or search our online titles: www.fs.fed.us/rm/publications www.treesearch.fs.fed.us/ Background This technical report provides a guide to assist arborists, landowners, woody plant pest management specialists, foresters, and plant pathologists in the diagnosis and control of tree diseases encountered in the Great Plains. It contains 56 chapters on tree diseases prepared by 27 authors, and emphasizes disease situations as observed in the 10 states of the Great Plains: Colorado, Kansas, Montana, Nebraska, New Mexico, North Dakota, Oklahoma, South Dakota, Texas, and Wyoming. The need for an updated tree disease guide for the Great Plains has been recog- nized for some time and an account of the history of this publication is provided here. -

Nematodes As Biocontrol Agents This Page Intentionally Left Blank Nematodes As Biocontrol Agents
Nematodes as Biocontrol Agents This page intentionally left blank Nematodes as Biocontrol Agents Edited by Parwinder S. Grewal Department of Entomology Ohio State University, Wooster, Ohio USA Ralf-Udo Ehlers Department of Biotechnology and Biological Control Institute for Phytopathology Christian-Albrechts-University Kiel, Raisdorf Germany David I. Shapiro-Ilan United States Department of Agriculture Agriculture Research Service Southeastern Fruit and Tree Nut Research Laboratory, Byron, Georgia USA CABI Publishing CABI Publishing is a division of CAB International CABI Publishing CABI Publishing CAB International 875 Massachusetts Avenue Wallingford 7th Floor Oxfordshire OX10 8DE Cambridge, MA 02139 UK USA Tel: þ44 (0)1491 832111 Tel: þ1 617 395 4056 Fax: þ44 (0)1491 833508 Fax: þ1 617 354 6875 E-mail: [email protected] E-mail: [email protected] Web site: www.cabi-publishing.org ßCAB International 2005. All rights reserved. No part of this publication may be reproduced in any form or by any means, electronically, mech- anically, by photocopying, recording or otherwise, without the prior permission of the copyright owners. A catalogue record for this book is available from the British Library, London, UK. Library of Congress Cataloging-in-Publication Data Nematodes as biocontrol agents / edited by Parwinder S. Grewal, Ralf- Udo Ehlers, David I. Shapiro-Ilan. p. cm. Includes bibliographical references and index. ISBN 0-85199-017-7 (alk. paper) 1. Nematoda as biological pest control agents. I. Grewal, Parwinder S. II. Ehlers, Ralf-Udo. III. Shaprio-Ilan, David I. SB976.N46N46 2005 632’.96–dc22 2004030022 ISBN 0 85199 0177 Typeset by SPI Publisher Services, Pondicherry, India Printed and bound in the UK by Biddles Ltd., King’s Lynn This volume is dedicated to Dr Harry K. -

Clearwing Moth PN 7477 Rev2
CLEARWING MOTHS Integrated Pest Management for Home Gardeners and Landscape Professionals The larvae of several species of clear- wing moths in the insect family Sesiidae are important wood-boring pests in landscapes. Hosts include (actual size) alder, ash, birch, fir, oak, pine, poplar, sycamore, willow, and stone fruit trees such as apricot, cherry, peach, and plum. Larvae that closely re- (actual semble those of clearwing moths in- adult egg size) clude the American plum borer (Euzophera semifuneralis, family (actual Pyralidae), a serious boring pest of size) hosts that include fruit and nut trees, pupa mountain ash, olive, and sycamore. Other common wood-boring pests in landscapes include bark beetles (fam- larvae ily Scolytidae) (for more information, see Pest Notes: Bark Beetles, listed in Suggested Reading), longhorned bor- ers (Cerambycidae), and round- headed borers (Buprestidae). (actual size) IDENTIFICATION Dying limbs, rough or gnarled bark, Figure 1. Clearwing moth stages and life cycle. and sawdustlike frass (insect excre- ment) are good indications that trees wider hind wings. The hind wings, pheromone that attracts males. After are infested with wood-boring in- and in some species the front wings, mating, the female deposits her tiny sects. When clearwing moth larvae are mostly clear. These moths fly dur- reddish to pale pink eggs in cracks, bore beneath tree bark, they push ing the day or at twilight, and their crevices, and rough or wounded areas frass from their tunnels; the frass is yellow and black coloring resembles on bark. Eggs hatch in about 1 to 4 sometimes mixed with gummy tree that of paper wasps or yellowjackets. -

Lilac/Ash Borer: a Common Wood Borer of Colorado’S Street Trees Fact Sheet No
Lilac/Ash Borer: A Common Wood Borer of Colorado’s Street Trees Fact Sheet No. 5.614 Insect Series|Trees and Shrubs by W.S. Cranshaw* Lilac/ash borer (Podosesia syringae1) is Quick Facts common wood borer associated with ash throughout Colorado and a species that is • Lilac/ash borer is an native to North America. Damage is caused insect native to North by the larvae which tunnel into the trunks America that is common and lower branches of ash trees. These in ash trees, particularly if feeding injuries produce irregular gouging trees are stressed due to wounds under the bark and tunneling drought, injury or that were frequently extends deeply into the heartwood recently transplanted. (Figure 1). Almost all larval feeding activity occurs in the lower trunk, particularly around • Damage is done by the larva; the soil line (Figure 2). Lower scaffold limbs a type of caterpillar that may also be attacked and infestations may tunnels into the trunk and extend about 10 feet up the trunk. Feeding lower branches of ash trees. damage can often be found in the mid and upper crown of trees above areas that were • If necessary, lilac/ash borer treated with contact insecticides. can be easily controlled by External evidence of lilac/ash borer spraying the trunk and lower activity in trees can include irregularly Figure 1. Lilac/ash borer larvae tunneling branches in spring during round exit holes of about 1/4-inch diameter exposed from under the bark. Photograph by the time when adult females David Leatherman. on trunks (Figure 3). -

Biodiversity and Coarse Woody Debris in Southern Forests Proceedings of the Workshop on Coarse Woody Debris in Southern Forests: Effects on Biodiversity
Biodiversity and Coarse woody Debris in Southern Forests Proceedings of the Workshop on Coarse Woody Debris in Southern Forests: Effects on Biodiversity Athens, GA - October 18-20,1993 Biodiversity and Coarse Woody Debris in Southern Forests Proceedings of the Workhop on Coarse Woody Debris in Southern Forests: Effects on Biodiversity Athens, GA October 18-20,1993 Editors: James W. McMinn, USDA Forest Service, Southern Research Station, Forestry Sciences Laboratory, Athens, GA, and D.A. Crossley, Jr., University of Georgia, Athens, GA Sponsored by: U.S. Department of Energy, Savannah River Site, and the USDA Forest Service, Savannah River Forest Station, Biodiversity Program, Aiken, SC Conducted by: USDA Forest Service, Southem Research Station, Asheville, NC, and University of Georgia, Institute of Ecology, Athens, GA Preface James W. McMinn and D. A. Crossley, Jr. Conservation of biodiversity is emerging as a major goal in The effects of CWD on biodiversity depend upon the management of forest ecosystems. The implied harvesting variables, distribution, and dynamics. This objective is the conservation of a full complement of native proceedings addresses the current state of knowledge about species and communities within the forest ecosystem. the influences of CWD on the biodiversity of various Effective implementation of conservation measures will groups of biota. Research priorities are identified for future require a broader knowledge of the dimensions of studies that should provide a basis for the conservation of biodiversity, the contributions of various ecosystem biodiversity when interacting with appropriate management components to those dimensions, and the impact of techniques. management practices. We thank John Blake, USDA Forest Service, Savannah In a workshop held in Athens, GA, October 18-20, 1993, River Forest Station, for encouragement and support we focused on an ecosystem component, coarse woody throughout the workshop process. -

Emerald Ash Borer Threatens Ash-Feeding Lepidoptera
News of the Lepidopterists’ Society Volume 49, Number 1 Conservation Matters: Contributions from the Conservation Committee With this column, we kick off one of the more visible products of the Society’s Conservation Committee – Conservation Matters. The double entendre speaks well to our goals for the column. We plan to bring conservation concerns and developments that impact our membership to the forefront. A forum for our committee members to discuss the issues, events, and research that excites and motivates them. But just as importantly, we hope to demonstrate that conservation does matter and that the decline of ecosystems and the Lepidoptera they support impact all of our interests. Collectors, watchers, researchers…, anyone and everyone with an interest in moths and butterflies is affected. It’s worth noting that this column will reflect various viewpoints from the committee. It won’t just include my own opinions in every issue, telling you over and over again about my particular concerns. Rather, we hope that as the column matures, it will truly reflect the full range of issues, threats and strategies that impact our beloved insects. Dave Wager’s kick-off for this column is exemplary – a unique assessment of an ecological threat that most are completely unaware of. As I’ve watched this particular disaster unfold right in my own back yard, attended strategy meetings with impacted stakeholders, and tried to find the funding required to push eradication during the initial discovery phase, I’ve been amazed by the lack of interest by the general population. I think Dave’s column brings the impact home to us – ash feeding specialist moths are doomed if the dire predictions are correct. -

Direct and Indirect Impacts of Emerald Ash Borer on Forest Bird
Direct and Indirect Impacts of Emerald Ash Borer on Forest Bird Communities THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Lawrence Charles Long Graduate Program in Entomology The Ohio State University 2013 Master's Examination Committee: Dr. Daniel A. Herms, Advisor Dr. Mary M. Gardiner Dr. Kathleen S. Knight Dr. Amanda D. Rodewald i Copyrighted by Lawrence Charles Long 2013 ii Abstract Emerald Ash Borer (EAB) (Agrilus planipennis Fairmare) is an exotic invader in North American forests. The buprestid wood borer has killed millions of ash trees since its discovery in southeastern Michigan in 2002. Because North American ash trees lack an evolutionary history with EAB, it has the potential to functionally extirpate the genus Fraxinus from the North American continent. Widespread, simultaneous ash mortality is likely to initiate a cascade of direct and indirect ecological effects. Forest infestation by EAB and subsequent loss of ash trees may lead to altered habitat and food availability for native insectivores such as birds. The objectives of this research were to: (1) determine utilization of EAB as a food resource by non-migratory bark-gleaning birds, (2) quantify forest regeneration in response to EAB-induced ash tree mortality and (3) determine the impact of EAB-induced regeneration on forest bird communities. Bird/vegetation monitoring plots were established during the winters of 2011 and 2012 in 51 forested stands throughout agricultural western and central Ohio as well as along the Huron river watershed in southeastern Michigan. During the spring of 2011 and 2012, point counts were conducted at each of the three plots per site. -

Emerald Ash Borer
Forest Health Technology Enterprise Team TECHNOLOGY TRANSFER Emerald Ash Borer Forest Health Technology Enterprise Team—Morgantown, West Virginia FHTET-2004-02 U.S. Department Forest Service Animal and Plant Health January 2004 of Agriculture Inspection Service Most of the abstracts were submitted in an electronic format, and were edited to achieve a uniform format and typeface. Each contributor is responsible for the accuracy and content of his or her own paper. Statements of the contributors from outside of the U.S. Department of Agriculture may not necessarily reflect the policy of the Department. Some participants did not submit abstracts, and so their presentations are not represented here. The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the U.S. Department of Agriculture of any product or service to the exclusion of others that may be suitable. Remarks about pesticides appear in some technical papers represented by these abstracts. Publication of these statements does not constitute endorsement or recommendation of them by the conference sponsors, nor does it imply that uses discussed have been registered. Use of most pesticides is regulated by state and federal laws. Applicable regulations must be obtained from the appropriate regulatory agency prior to their use. CAUTION: Pesticides can be injurious to humans, domestic animals, desirable plants, and fish and other wildlife if they are not handled and applied properly. Use all pesticides selectively and carefully. Follow recommended practices given on the label for use and disposal of pesticides and pesticide containers. -
Moths of North Carolina - Early Draft 1
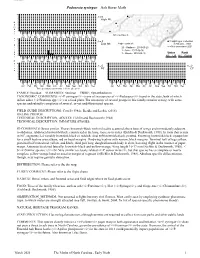
Sesiidae Podosesia syringae Ash Borer Moth 10 9 8 n=0 • 7 High Mt. • 6 N 5 • u 4 3 • • m 2 • b 1 e 0 • r 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 • 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 NC counties: 8 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec o 10 f 9 n=5 = Sighting or Collection 8 • 7 Low Mt. High counts of: in NC since 2001 F 6 l 5 20 - Madison - 2019-05-25 = Not seen since 2001 4 • i 3 1 - Ashe - 2017-06-06 g 2 Status Rank h 1 1 - Macon - 2018-05-15 0 NC US NC Global t 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 D Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec a 10 10 9 9 t 8 n=0 8 n=0 e 7 Pd 7 CP s 6 6 5 5 4 4 3 3 2 2 1 1 0 0 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 15 5 25 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Three periods to each month: 1-10 / 11-20 / 21-31 FAMILY: Sesiidae SUBFAMILY: Sesiinae TRIBE: Synanthedonini TAXONOMIC_COMMENTS: <i>P. -

A Survey of Key Arthropod Pests on Common Southeastern Street Trees
Arboriculture & Urban Forestry 45(5): September 2019 155 Arboriculture & Urban Forestry 2019. 45(5):155–166 ARBORICULTURE URBAN FORESTRY Scientific Journal of the International& Society of Arboriculture A Survey of Key Arthropod Pests on Common Southeastern Street Trees By Steven D. Frank Abstract. Cities contain dozens of street tree species each with multiple arthropod pests. Developing and implementing integrated pest management (IPM) tactics, such as scouting protocols and thresholds, for all of them is untenable. A survey of university research and extension personnel and tree care professionals was conducted as a first step in identifying key pests of common street tree genera in the Southern United States. The survey allowed respondents to rate seven pest groups from 0 (not pests) to 3 (very important or damaging) for each of ten tree genera. The categories were sucking insects on bark, sucking insects on leaves, defoliators and leafminers, leaf and stem gall forming arthropods, trunk and twig borers and bark beetles, and mites. Respondents could also identify important pest species within categories. Some tree genera, like Quercus and Acer, have many important pests in multiple categories. Other genera like Lirioden- dron, Platanus, and Lagerstroemia have only one or two key pests. Bark sucking insects were the highest ranked pests of Acer spp. Defo- liators, primarily caterpillars, were ranked highest on Quercus spp. followed closely by leaf and stem gallers, leaf suckers, and bark suckers. All pest groups were rated below ‘1’ on Zelkova spp. Identifying key pests on key tree genera could help researchers prioritize IPM development and help tree care professionals prioritize their training and IPM implementation. -
Phylogenomics Reveals the Evolutionary Timing and Pattern of Butterflies and Moths
Phylogenomics reveals the evolutionary timing and pattern of butterflies and moths Akito Y. Kawaharaa,1, David Plotkina,b, Marianne Espelanda,c, Karen Meusemannd,e,f, Emmanuel F. A. Toussainta,g, Alexander Donathe, France Gimniche, Paul B. Frandsenh,i, Andreas Zwickf, Mario dos Reisj, Jesse R. Barberk, Ralph S. Petersc, Shanlin Liul,m, Xin Zhoum, Christoph Mayere, Lars Podsiadlowskie, Caroline Storera, Jayne E. Yackn, Bernhard Misofe, and Jesse W. Breinholta,o,2 aMcGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, Gainesville, FL 32611; bEntomology and Nematology Department, University of Florida, Gainesville, FL 32611; cArthropoda Department, Alexander Koenig Zoological Research Museum, D-53113 Bonn, Germany; dDepartment of Evolutionary Biology and Ecology, Institute for Biology I (Zoology), University of Freiburg, D-79104 Freiburg, Germany; eCenter for Molecular Biodiversity Research, Alexander Koenig Zoological Research Museum, D-53113 Bonn, Germany; fAustralian National Insect Collection, National Research Collections Australia, Commonwealth Scientific and Industrial Research Organisation, Canberra, Acton, ACT 2601, Australia; gDepartment of Entomology, Natural History Museum of Geneva, 1208 Geneva, Switzerland; hPlant and Wildlife Sciences, Brigham Young University, Provo, UT 84602; iData Science Lab, Smithsonian Institution, Washington, DC 20002; jSchool of Biological and Chemical Sciences, Queen Mary University of London, London E1 4NS, United Kingdom; kDepartment of Biological