A Comprehensive Review of Genetics and Genetic Testing in Azoospermia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Case Report Full Text Online At
Case Report Full text online at http://www.jiaps.com Penile agenesis A. K. Bangroo, Ramji Khetri, Sashi Tiwari St Stephen's Hospital, Tis Hazari, Delhi Correspondence: AK Bangroo, 103, Administrative block, St. Stephens Hospital, Tis Hazari, Delhi-110054, India. E-mail: [email protected] ABSTRACT Penile agenesis is an extremely rare disorder with profound urological and psychological consequences. The goal of treatment is an early female gender assignment and feminizing reconstruction of the perineum. KEY WORDS: Aphallia, Penile agenesis, Ambiguous genitalia Penile agenesis (PA) is an extremely rare developmental the scrotal folds which were preserved for subsequent anomaly with the reported incidence of 1 in 30 million genital reconstruction. births[1]. PA is believed to result from either the absence of the genital tubercle, or its failure to develop.[2] Several DISCUSSION investigators claim the absence of corpora cavernosa and corpora spongiosum as a prerequisite for the diagnosis of The earliest case report of aphallia was by Imminger in penile agenesis.[3] Except for the reported XX-XY mosaic, 1853[2] since then only 75 cases have been reported in the patients have 46 XY karyotypes.[4] More than half of these literature[6]. Skoog and Belman[5] suggested three variants, have associated anomalies, including developmental de based on urethral position in relationship to the anal fects of the caudal axis, genitourinary and gastrointestinal sphincter, as: Postsphincteric; Presphincteric tract anomalies.[5] The scrotum, testes and testicular func (Prostatorectal fistula) and Urethral atresia. More proxi tion are usually normal[2]. mal the bladder outlet, greater is the likelihood of other anomalies and death.[5] CASE REPORT A two-day-old 3.2 kg genotypic male (46XY) neonate was brought, by a social organization, to our hospital with the complaint of absence of penis, and passage of meco nium mixed with urine through rectum. -

A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years
Journal of Advances in Radiology and Medical Imaging Volume 4 | Issue 1 ISSN: 2456-5504 Case Report Open Access A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years Old Man D’Amato D*, Ranalli T, Tatulli D, Bocchinfuso F, Manenti G, Valente F and Bizzaglia M Diagnostic and Interventional Radiology, Policlinico Tor Vergata, University of Rome “Tor Vergata”, Rome, Italy *Corresponding author: D’Amato D, Diagnostic and Interventional Radiology, Policlinico Tor Vergata, University of Rome “Tor Vergata”, Viale Oxford 181, Rome, Italy, Tel: +393207034690, E-mail: [email protected] Citation: D’Amato D, Ranalli T, Tatulli D, Bocchinfuso F, Manenti G, et al. (2019) A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years Old Man. J Adv Radiol Med Image 4(1): 102 Received Date: April 04, 2019 Accepted Date: August 26, 2019 Published Date: August 28, 2019 Abstract Congenital anorchia is an uncommon clinical condition. Etiology and pathogenetic mechanisms are often unknown. Although some patients with anorchia present with ambiguous external genitalia or micropenis, most have a normal phenotype. XY Disorders of Sex Development classifications are numerous and success rate in establishing a precise diagnosis is far lower than in XX karyotype. We report the case of a young man, with 46 XY karyotype showing various uro-genital abnormalities. A definitive diagnosis was not established due to the complex clinical presentation. Ultrasonography and Magnetic Resonance Imaging techniques were useful tools in the definition of uro-genital anomalies and gonadal development in this complex scenario. Keywords: Anorchia; Cryptorchidism; Urogenital Anomalies; DSD; MRI List of abbreviations: MRI: Magnetic Resonance Imaging; US: Ultrasonography; DSD: Disorders of Sex Development, FSH: Follicle- Stimulating Hormone; HCG: Human Chorionic Gonadotropin; LH: Luteinizing Hormone; AMH Antimüllerian Hormone; LHRH LH- Releasing Hormone; SD: Standard Deviation Introduction The disorders of sexual differentiation constitute a challenging area for both diagnostic and therapeutic impact. -

Growth Hormone Deficiency Causing Micropenis:Peter A
Growth Hormone Deficiency Causing Micropenis:Peter A. Lee, MD, PhD, a Tom Mazur, PsyD, Lessons b Christopher P. Houk, MD, Learned c Robert M. Blizzard, MD d From a Well-Adjusted Adultabstract This report of a 46, XY patient born with a micropenis consistent with etiology from isolated congenital growth hormone deficiency is used to (1) raise the question regarding what degree testicular testosterone exposure aDepartment of Pediatrics, College of Medicine, Penn State to the central nervous system during fetal life and early infancy has on the University, Hershey, Pennsylvania; bCenter for Psychosexual development of male gender identity, regardless of gender of rearing; (2) Health, Jacobs School of Medicine and Biomedical suggest the obligatory nature of timely full disclosure of medical history; Sciences, University at Buffalo and John R. Oishei Children’s Hospital, Buffalo, New York; cDepartment of (3) emphasize that virtually all 46, XY infants with functional testes and Pediatrics, Medical College of Georgia, Augusta University, a micropenis should be initially boys except some with partial androgen Augusta, Georgia; and dDepartment of Pediatrics, College of Medicine, University of Virginia, Charlottesville, Virginia insensitivity syndrome; and (4) highlight the sustaining value of a positive long-term relationship with a trusted physician (R.M.B.). When this infant Dr Lee reviewed and discussed the extensive medical records with Dr Blizzard, reviewed presented, it was commonly considered inappropriate to gender assign an pertinent medical literature, and wrote each draft infant male whose penis was so small that an adult size was expected to be of the manuscript with input from all coauthors; inadequate, even if the karyotype was 46, XY, and testes were functional. -
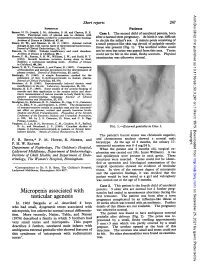
Micropenis Associated with Testicular Agenesis
Arch Dis Child: first published as 10.1136/adc.50.3.247 on 1 March 1975. Downloaded from Short reports 247 REFERENCES Patients Barnes, N. D., Joseph, J. M., Atherden, S. M. and Clayton, B. E. (1972). Functional tests of adrenal axis in children with Case 1. The second child of unrelated parents, born measurement of plasma cortisol by competitive protein binding. after a normal term pregnancy. At birth it was difficult Archives of Disease in Childhood, 47, 66. to decide the infant's sex. A minute penis consisting of Deane, H. W., and Masson, G. M. C. (1951). Adrenal cortical a small prepuce-like skin tag devoid of palpable erectile changes in rats with various types of experimental hypertension. .ournal of Clinical Endocrinology, 11, 193. tissue was present (Fig. 1). The urethral orifice could Fanconi, G. (1954). Tubular insufficiency and renal dwarfism. not be seen but urine was passed from this area. Testes Archives of Disease in Childhood, 29, 1. could not be felt in the small, fleshy scrotum. Physical Howse, P. M., Rayner, P. H. W., Williams, J. W., and Rudd, B. T. (1974). Growth hormone secretion during sleep in short examination was otherwise normal. children: a continuous sampling study. Archives of Disease in Childhood, 49, 246. James, V. H. T., Townsend, J., and Fraser, R. (1967). Comparison of fluorimetric and isotopic procedures for the determination of plasma cortisol. journal of Endocrinology, 37, xxviii. Mattingly, D. (1962). A simple fluorimetric method for the estimation of free 1 1-hydroxycorticoids in human plasma. Journal of Clinical Pathology, 15, 374. -

Pathogenic Variants in the DEAH-Box RNA Helicase DHX37 Are a Frequent Cause of 46,XY Gonadal Dysgenesis and 46,XY Testicular Regression Syndrome
ARTICLE © American College of Medical Genetics and Genomics Pathogenic variants in the DEAH-box RNA helicase DHX37 are a frequent cause of 46,XY gonadal dysgenesis and 46,XY testicular regression syndrome Ken McElreavey, PhD 1, Anne Jorgensen, PhD 2, Caroline Eozenou, PhD1, Tiphanie Merel, MSc1, Joelle Bignon-Topalovic, BSc1, Daisylyn Senna Tan, BSc3, Denis Houzelstein, PhD 1, Federica Buonocore, PhD 4, Nick Warr, PhD 5, Raissa G. G. Kay, PhD5, Matthieu Peycelon, MD, PhD 6,7,8, Jean-Pierre Siffroi, MD, PhD6, Inas Mazen, MD9, John C. Achermann, MD, PhD 4, Yuliya Shcherbak, MD10, Juliane Leger, MD, PhD11, Agnes Sallai, MD 12, Jean-Claude Carel, MD, PhD 11, Laetitia Martinerie, MD, PhD11, Romain Le Ru, MD13, Gerard S. Conway, MD, PhD14, Brigitte Mignot, MD15, Lionel Van Maldergem, MD, PhD 16, Rita Bertalan, MD, PhD17, Evgenia Globa, MD, PhD 18, Raja Brauner, MD, PhD19, Ralf Jauch, PhD 3, Serge Nef, PhD 20, Andy Greenfield, PhD5 and Anu Bashamboo, PhD1 Purpose: XY individuals with disorders/differences of sex devel- specifically associated with gonadal dysgenesis and TRS. opment (DSD) are characterized by reduced androgenization Consistent with a role in early testis development, DHX37 is caused, in some children, by gonadal dysgenesis or testis regression expressed specifically in somatic cells of the developing human during fetal development. The genetic etiology for most patients and mouse testis. with 46,XY gonadal dysgenesis and for all patients with testicular Conclusion: DHX37 pathogenic variants are a new cause of an regression syndrome (TRS) is unknown. autosomal dominant form of 46,XY DSD, including gonadal Methods: We performed exome and/or Sanger sequencing in 145 dysgenesis and TRS, showing that these conditions are part of a individuals with 46,XY DSD of unknown etiology including clinical spectrum. -

Genetics of Azoospermia
International Journal of Molecular Sciences Review Genetics of Azoospermia Francesca Cioppi , Viktoria Rosta and Csilla Krausz * Department of Biochemical, Experimental and Clinical Sciences “Mario Serio”, University of Florence, 50139 Florence, Italy; francesca.cioppi@unifi.it (F.C.); viktoria.rosta@unifi.it (V.R.) * Correspondence: csilla.krausz@unifi.it Abstract: Azoospermia affects 1% of men, and it can be due to: (i) hypothalamic-pituitary dysfunction, (ii) primary quantitative spermatogenic disturbances, (iii) urogenital duct obstruction. Known genetic factors contribute to all these categories, and genetic testing is part of the routine diagnostic workup of azoospermic men. The diagnostic yield of genetic tests in azoospermia is different in the different etiological categories, with the highest in Congenital Bilateral Absence of Vas Deferens (90%) and the lowest in Non-Obstructive Azoospermia (NOA) due to primary testicular failure (~30%). Whole- Exome Sequencing allowed the discovery of an increasing number of monogenic defects of NOA with a current list of 38 candidate genes. These genes are of potential clinical relevance for future gene panel-based screening. We classified these genes according to the associated-testicular histology underlying the NOA phenotype. The validation and the discovery of novel NOA genes will radically improve patient management. Interestingly, approximately 37% of candidate genes are shared in human male and female gonadal failure, implying that genetic counselling should be extended also to female family members of NOA patients. Keywords: azoospermia; infertility; genetics; exome; NGS; NOA; Klinefelter syndrome; Y chromosome microdeletions; CBAVD; congenital hypogonadotropic hypogonadism Citation: Cioppi, F.; Rosta, V.; Krausz, C. Genetics of Azoospermia. 1. Introduction Int. J. Mol. Sci. -

Surgical Correction of a Penoscrotal Web:A Report of a Case With
www.symbiosisonline.org Symbiosis www.symbiosisonlinepublishing.com Review Article SOJ Surgery Open Access Surgical Correction of a Penoscrotal Web:A Report of a Case with Literature Review Volkan Sarper Erikci1*, Merve Dilara Öney2, Gökhan Köylüoğlu3 1Attending Pediatric Surgeon, Associate Professor of Pediatric Surgery, Sağlık Bilimleri University, TURKEY 2Trainee in Pediatric Surgery, Sağlık Bilimleri University,Turkey 3Professor of Pediatric Surgery, Chief Department of Pediatric Surgery, Katip Çelebi University, Turkey Received: 7 July, 2017; Accepted: 14 September, 2017; Published: 23 September, 2017 *Corresponding author: Volkan Sarper Erikci, Attending Pediatric Surgeon, Associate Professor of Pediatric Surgery, Sağlık Bilimleri University, Kazim Dirik Mah Mustafa Kemal Cad Hakkibey apt. No:45 D.10 35100 Bornova-İzmir. GSM: +90 542 4372747, Business phone: +90 232 4696969, Fax: +90 232 4330756; E-mail: [email protected] and the medical history did not reveal local infection, urinary Abstract retention or chronic urinary dripping. But the parents were Penoscrotal Webbing (PSW) is a penile and scrotal skin anxious because they felt that their child’s penis was too short. In abnormality that is considered in the spectrum of buried penis. Various surgical techniques have been proposed for PSW with on the ventral aspect of the penis solved the problem (Figure 3,4). different terminologies. Herein we present a 7-year-old boy with PSW Withaddition an uneventful to circumcision, postoperative foreskin period,reconstruction the family -

Prader Willi Syndrome (PWS) and Hypogonadism
4/28/2015 Prader Willi Syndrome and Hypogonadism Kathryn Anglin, MSN, BSN, RN Pediatric Endocrine Clinical Nurse Specialist Nationwide Children’s Hospital Columbus, Ohio ………………..…………………………………………………………………………………………………………………………………….. No Conflict of Interest to Disclose ………………..…………………………………………………………………………………………………………………………………….. Objectives • Identify the clinical features of hypogonadism and incomplete / delayed puberty in a male with Prader Willi syndrome (PWS) • Understand the role of hCG in evaluation and treatment of hypogonadism in PWS • Discuss expert recommendations for the treatment of hypogonadism in males with PWS 1 4/28/2015 Introduction • PWS is a multisystem genetic disorder (15q11.2-q13) • Complex phenotype likely caused by hypothalamic dysfunction leading to hormonal dysfunction and the absence of satiety • Hypotonia and hypogonadism are the first manifestations of a primitive hypothalammic alteration, which many believe is the basis of PWS Introduction • Hypogonadism is a common clinical feature of PWS which confirms the importance of hypogonadism as a major diagnostic criterion of PWS • Patients with PWS commonly fail to spontaneously initiate or complete puberty • However, many have premature adrenarche • Precocious puberty is more rare Case Study Hypogonadisn in PWS Currently 19 year old male History: • Diagnosed clinically at age 2 years, and at 6 years based on methylation studies; Consistent with imprinting abnormality* • Hypotonia and poor feeding in the newborn period Developmental delay and hyperphagia in the early -

The Approach to the Infant with Ambiguous Genitalia
334 Review Article Disorders/differences of sex development (DSDs) for primary care: the approach to the infant with ambiguous genitalia Justin A. Indyk Section of Endocrinology, Nationwide Children’s Hospital, the Ohio State University, Columbus, Ohio 43205, USA Correspondence to: Justin A. Indyk, MD, PhD. THRIVE Program, Section of Endocrinology, Nationwide Children’s Hospital, 700 Children’s Drive, Columbus, Ohio 43205, USA. Email: [email protected]. Abstract: The initial management of the neonate with ambiguous genitalia can be a very stressful and anxious time for families, as well as for the general practitioner or neonatologist. A timely approach must be sensitive and attend to the psychosocial needs of the family. In addition, it must also effectively address the diagnostic dilemma that is frequently seen in the care of patients with disorders of sex development (DSDs). One great challenge is assigning a sex of rearing, which must take into account a variety of factors including the clinical, biochemical and radiologic clues as to the etiology of the atypical genitalia (AG). However, other important aspects cannot be overlooked, and these include parental and cultural views, as well as the future outlook in terms of surgery and fertility potential. Achieving optimal outcomes requires open and transparent dialogue with the family and caregivers, and should harness the resources of a multidisciplinary team. The multiple facets of this approach are outlined in this review. Keywords: Sex; gender; genitalia; DSD; -

Benign Penile Skin Anomalies in Children: a Primer for Pediatricians
Penile skin anomalies in children Benign penile skin anomalies in children: a primer for pediatricians Marco Castagnetti, Mike Leonard, Luis Guerra, Ciro Esposito, Marcello Cimador Padua, Italy Background: Abnormalities involving the skin evidence based choices for management, and recognize coverage of the penis are diffi cult to defi ne, but they can complications or untoward outcomes in patients significantly alter penile appearance, and be a cause of undergoing surgery. Review article parental concern. World J Pediatr March 2015; Online First Data sources: The present review was based on a non- Key words: balanitis xerotica obliterans; systematic search of the English language medical literature foreskin; using a combination of key words including "penile penis; skin anomalies" and the specific names of the different phimosis conditions. Results: Conditions were addressed in the following order, those mainly affecting the prepuce (phimosis, balanitis xerotica obliterans, balanitis, paraphimosis), Introduction those which alter penile configuration (inconspicuous enile anomalies are quite common in children and are penis and penile torsion), and lastly focal lesions (cysts, almost invariably a cause of concern for their parents. nevi and vascular lesions). Most of these anomalies are PSome of them, such as hypospadias or epispadias congenital, have no or minimal influence on urinary are clearly of surgical interest and specific reviews exist [1-3] function, and can be detected on clinical examination. regarding their management. Abnormalities involving Spontaneous improvement is possible. In the majority the skin coverage of the penis can be more difficult to of cases undergoing surgery, the potential psychological define. In most of these cases, there is minimal or no implications of genital malformation on patient impact on urinary function, but penile appearance can be development are the main reason for treatment, and signifi cantly altered. -

Hypomethylation of the DAZ3 Promoter in Idiopathic Asthenospermia: a Screening Tool for Liquid Biopsy Shichang Zhang1,3, Li Xu2,3, Mengyao Yu1 & Jiexin Zhang1*
www.nature.com/scientificreports OPEN Hypomethylation of the DAZ3 promoter in idiopathic asthenospermia: a screening tool for liquid biopsy Shichang Zhang1,3, Li Xu2,3, Mengyao Yu1 & Jiexin Zhang1* Given the role of the deleted in azoospermia gene in male infertility, whether the somatic deleted in azoospermia methylation status is associated with idiopathic asthenospermia should be determined. To investigate the methylation levels of the deleted in azoospermia promoter in peripheral white blood cells from idiopathic asthenospermia patients relative to those in normozoospermia controls, 61 ethylene diamine tetraacetic acid anticoagulant blood samples were drawn from all participants for DNA isolation. The deleted in azoospermia promoter methylation ratio was detected by MassARRAY-based methylation quantifcation and confrmed by quantitative methylation-specifc polymerase chain reaction. A MassARRAY-based methylation analysis showed that the deleted in azoospermia 3 promoter (0 to − 2 kbp) was signifcantly hypomethylated in peripheral white blood cells from idiopathic asthenospermia males, specifcally one CpG site (− 246 to − 247). Quantitative methylation-specifc polymerase chain reaction data further confrmed that the methylation level of the deleted in azoospermia 3 promoter region in idiopathic asthenospermia patients was signifcantly lower than that in normozoospermia males. The area under the receiver operating characteristic curve determined by quantitative methylation-specifc polymerase chain reaction was 0.737 (95% confdence interval: 0.552 to 0.924), with a sensitivity of 53.9% and a specifcity of 88.2% at a cut- of level of 74.7%. Therefore, our results suggested that methylation ratio detection of the deleted in azoospermia 3 promoter region by real-time polymerase chain reaction assay is a promising and feasible tool for liquid biopsy in the clinical laboratories. -

910. Ida Bagus Andhita Male Pseudohermaphroditism 236-.P65
Paediatrica Indonesiana VOLUME 46 September - October • 2006 NUMBER 9-10 Case Report Male pseudohermaphroditism due to 5-alpha reductase type-2 deficiency in a 20-month old boy Ida Bagus Andhita, Wayan Bikin Suryawan ntersex conditions are the most fascinating con- paper reports a 20-month old patient with male ditions encountered by clinicians. The ability pseudohermaphroditism due to 5-alpha reductase to diagnose infants born with this disorder has type-2 deficiency. Iadvanced rapidly in recent years. In most cases, clinicians can promptly make an accurate diagnosis and give the advice to the parents on therapeutic Report of the case options. Intersex conditions traditionally have been divided into the following 5 simplified classifications A 20-month old ”girl”, came to the outpatient clinic based on the differentiation of the gonad, i.e. 1) fe- of the Department of Child Health, Sanglah Hospi- male pseudohermaphrodite characterized by two tal, Denpasar, with the chief complaint of a bump on ovaries, 2) male pseudohermaphrodite characterized the urinary duct noted since three months before ad- by two testes, 3) true hermaphrodite characterized mission. The urination and defecation were normal. by ovary and or testis and or ovotestis, 4) mixed go- History of pregnancy and delivery were normal. There nadal dysgenesis characterized by testis plus streak was no history of the same condition among the fam- gonad, and 5) pure gonadal dysgenesis characterized ily. No history of oral contraceptive, alcohol intake, by bilateral streak gonads.1-3 hormonal, or traditional medication during pregnancy. 5-alpha-reductase (5-ARD) type 2 deficiency His growth and development were normal.