Molten Salt Energy Storage System for DEMO Operated in Pulsed Mode
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

High Temperature Enthalpies of the Lead Halides
HIGH TEMPERATURE ENTHALPIES OF THE LEAD HALIDES: ENTHALPIES AND ENTROPIES OF FUSION APPROVED: Graduate Committee: Major Professor Committee Member Min fessor Committee Member X Committee Member UJ. Committee Member Committee Member Chairman of the Department of Chemistry Dean or the Graduate School HIGH TEMPERATURE ENTHALPIES OF THE LEAD HALIDES: ENTHALPIES AND ENTROPIES OF FUSION DISSERTATION Presented to the Graduate Council of the North Texas State University in Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY By Clarence W, Linsey, B. S., M. S, Denton, Texas June, 1970 ACKNOWLEDGEMENT This dissertation is based on research conducted at the Oak Ridge National Laboratory, Oak Ridge, Tennessee, which is operated by the Uniofl Carbide Corporation for the U. S. Atomic Energy Commission. The author is also indebted to the Oak Ridge Associated Universities for the Oak Ridge Graduate Fellowship which he held during the laboratory phase. 11 TABLE OF CONTENTS Page LIST OF TABLES iv LIST OF ILLUSTRATIONS v Chapter I. INTRODUCTION . 1 Lead Fluoride Lead Chloride Lead Bromide Lead Iodide II. EXPERIMENTAL 11 Reagents Fusion-Filtration Method Encapsulation of Samples Equipment and Procedure Bunsen Ice Calorimeter Computer Programs Shomate Method Treatment of Data Near the Melting Point Solution Calorimeter Heat of Solution Calculations III. RESULTS AND DISCUSSION 41 Lead Fluoride Variation in Heat Contents of the Nichrome V Capsules Lead Chloride Lead Bromide Lead Iodide Summary BIBLIOGRAPHY ...... 98 in LIST OF TABLES Table Pa8e I. High-Temperature Enthalpy Data for Cubic PbE2 Encapsulated in Gold 42 II. High-Temperature Enthalpy Data for Cubic PbF2 Encapsulated in Molybdenum 48 III. -

Specific Energy Limit and Its Influence on the Nature of Black Holes Javier Viaña
Specific Energy Limit and its Influence on the Nature of Black Holes Javier Viaña To cite this version: Javier Viaña. Specific Energy Limit and its Influence on the Nature of Black Holes. 2021. hal- 03322333 HAL Id: hal-03322333 https://hal.archives-ouvertes.fr/hal-03322333 Preprint submitted on 19 Aug 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 16th of August of 2021 Specific Energy Limit and its Influence on the Nature of Black Holes Javier Viaña [0000-0002-0563-784X] University of Cincinnati, Cincinnati OH 45219, USA [email protected] What if the universe has a limit on the amount of energy that a certain mass can have? This article explores this possibility and suggests a theory for the creation and nature of black holes based on an energetic limit. The Specific Energy Limit Energy is an extensive property, and we know that as we add more mass to a given system, we can easily increase its energy. Specific energy on the other hand is an intensive property. It is defined as the energy divided by the mass and it is measured in units of J/kg. -

Chapter 11 Liquids, Solids, and Phase Changes
Lecture Presentation Chapter 11 Liquids, Solids, and Phase Changes 11.1, 11.2, 11.3, 11.4, 11.15, 11.17, 11.26, 11.30, 11.32, 11.40, 11.42, 11.60, 11.82, 11.116 John E. McMurry Robert C. Fay Properties of Liquids Viscosity: The measure of a liquid’s resistance to flow (higher intermolecular force, higher viscosity) Surface Tension: The resistance of a liquid to spread out and increase its surface area (forms beads) (higher intermolecular force, higher surface tension) Properties of Liquids low intermolecular force – low viscosity, low surface tension high intermolecular force – high viscosity, high surface tension HW 11.1 Properties of Liquids high intermolecular force – high viscosity, high surface tension For the following molecules which has the higher intermolecular force, viscosity and surface tension ? (LD structure, VSEPRT, dipole of molecule) (if the molecules are in the liquid state) a. CHCl3 vs CH4 b. H2O vs H2 (changed from handout) c. N Cl3 vs N H Cl2 Phase Changes between Solids, Liquids, and Gases Phase Change (State Change): A change in the physical state but not the chemical identity of a substance Fusion (melting): solid to liquid Freezing: liquid to solid Vaporization: liquid to gas Condensation: gas to liquid Sublimation: solid to gas Deposition: gas to solid Phase Changes between Solids, Liquids, and Gases Enthalpy – add heat to system Entropy – add randomness to system Phase Changes between Solids, Liquids, and Gases Heat (Enthalpy) of Fusion (DHfusion ): The amount of energy required to overcome enough intermolecular forces to convert a solid to a liquid Heat (Enthalpy) of Vaporization (DHvap): The amount of energy required to overcome enough intermolecular forces to convert a liquid to a gas HW 11.2: Phase Changes between Solids, Liquids, & Gases DHfusion solid to a liquid DHvap liquid to a gas At phase change (melting, boiling, etc) : DG = DH – TDS & D G = zero (bc 2 phases in equilibrium) DH = TDS o a. -

Energy and the Hydrogen Economy
Energy and the Hydrogen Economy Ulf Bossel Fuel Cell Consultant Morgenacherstrasse 2F CH-5452 Oberrohrdorf / Switzerland +41-56-496-7292 and Baldur Eliasson ABB Switzerland Ltd. Corporate Research CH-5405 Baden-Dättwil / Switzerland Abstract Between production and use any commercial product is subject to the following processes: packaging, transportation, storage and transfer. The same is true for hydrogen in a “Hydrogen Economy”. Hydrogen has to be packaged by compression or liquefaction, it has to be transported by surface vehicles or pipelines, it has to be stored and transferred. Generated by electrolysis or chemistry, the fuel gas has to go through theses market procedures before it can be used by the customer, even if it is produced locally at filling stations. As there are no environmental or energetic advantages in producing hydrogen from natural gas or other hydrocarbons, we do not consider this option, although hydrogen can be chemically synthesized at relative low cost. In the past, hydrogen production and hydrogen use have been addressed by many, assuming that hydrogen gas is just another gaseous energy carrier and that it can be handled much like natural gas in today’s energy economy. With this study we present an analysis of the energy required to operate a pure hydrogen economy. High-grade electricity from renewable or nuclear sources is needed not only to generate hydrogen, but also for all other essential steps of a hydrogen economy. But because of the molecular structure of hydrogen, a hydrogen infrastructure is much more energy-intensive than a natural gas economy. In this study, the energy consumed by each stage is related to the energy content (higher heating value HHV) of the delivered hydrogen itself. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Superconducting Magnetic Energy Storage and Superconducting Self-Supplied Electromagnetic Launcher★
Eur. Phys. J. Appl. Phys. 80, 20901 (2017) THE EUROPEAN © EDP Sciences, 2017 PHYSICAL JOURNAL DOI: 10.1051/epjap/2017160452 APPLIED PHYSICS Regular Article Superconducting magnetic energy storage and superconducting self-supplied electromagnetic launcher★ Jérémie Ciceron*, Arnaud Badel, and Pascal Tixador Institut Néel, G2ELab CNRS/Université Grenoble Alpes, Grenoble, France Received: 5 December 2016 / Received in final form: 8 April 2017 / Accepted: 16 August 2017 Abstract. Superconductors can be used to build energy storage systems called Superconducting Magnetic Energy Storage (SMES), which are promising as inductive pulse power source and suitable for powering electromagnetic launchers. The second generation of high critical temperature superconductors is called coated conductors or REBCO (Rare Earth Barium Copper Oxide) tapes. Their current carrying capability in high magnetic field and their thermal stability are expanding the SMES application field. The BOSSE (Bobine Supraconductrice pour le Stockage d’Energie) project aims to develop and to master the use of these superconducting tapes through two prototypes. The first one is a SMES with high energy density. Thanks to the performances of REBCO tapes, the volume energy and specific energy of existing SMES systems can be surpassed. A study has been undertaken to make the best use of the REBCO tapes and to determine the most adapted topology in order to reach our objective, which is to beat the world record of mass energy density for a superconducting coil. This objective is conflicting with the classical strategies of superconducting coil protection. A different protection approach is proposed. The second prototype of the BOSSE project is a small-scale demonstrator of a Superconducting Self-Supplied Electromagnetic Launcher (S3EL), in which a SMES is integrated around the launcher which benefits from the generated magnetic field to increase the thrust applied to the projectile. -

Specific Energy
Quantum Mechanics_Specific energy Specific energy SI unit J/kg In SI base units m2/s2 Derivations from other quantities e = E/m Energy density has tables of specific energies of devices and materials. Specific energy is energy per unit mass. (It is also sometimes called "energy density," though "energy density" more precisely means energy per unit volume.) It is used to quantify, for example, stored heat or other thermodynamic propertiesof substances such as specific internal energy, specific enthalpy, specific Gibbs free energy, and specific Helmholtz free energy. It may also be used for the kinetic energy or potential energy of a body. Specific energy is an intensive property, whereas energy and mass are extensive properties. The SI unit for specific energy is the joule per kilogram (J/kg). Other units still in use in some contexts are the kilocalorie per gram (Cal/g or kcal/g), mostly in food-related topics, watt hours per kilogram in the field of batteries, and theImperial unit BTU per pound (BTU/lb), in some engineering and applied technical fields.[1] The gray and sievert are specialized measures for specific energy absorbed by body tissues in the form of radiation. The following table shows the factors for converting to J/kg: Unit SI equivalent kcal/g[2] 4.184 MJ/kg Wh/kg 3.6 kJ/kg kWh/kg 3.6 MJ/kg Btu/lb[3] 2.326 kJ/kg Btu/lb[4] ca. 2.32444 kJ/kg The concept of specific energy is related to but distinct from the chemical notion of molar energy, that is energy per mole of a substance. -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -

From Cell to Battery System in Bevs: Analysis of System Packing Efficiency and Cell Types
Article From Cell to Battery System in BEVs: Analysis of System Packing Efficiency and Cell Types Hendrik Löbberding 1,* , Saskia Wessel 2, Christian Offermanns 1 , Mario Kehrer 1 , Johannes Rother 3, Heiner Heimes 1 and Achim Kampker 1 1 Chair for Production Engineering of E-Mobility Components, RWTH Aachen University, 52064 Aachen, Germany; c.off[email protected] (C.O.); [email protected] (M.K.); [email protected] (H.H.); [email protected] (A.K.) 2 Fraunhofer IPT, 48149 Münster, Germany; [email protected] 3 Faculty of Mechanical Engineering, RWTH Aachen University, 52072 Aachen, Germany; [email protected] * Correspondence: [email protected] Received: 7 November 2020; Accepted: 4 December 2020; Published: 10 December 2020 Abstract: The motivation of this paper is to identify possible directions for future developments in the battery system structure for BEVs to help choosing the right cell for a system. A standard battery system that powers electrified vehicles is composed of many individual battery cells, modules and forms a system. Each of these levels have a natural tendency to have a decreased energy density and specific energy compared to their predecessor. This however, is an important factor for the size of the battery system and ultimately, cost and range of the electric vehicle. This study investigated the trends of 25 commercially available BEVs of the years 2010 to 2019 regarding their change in energy density and specific energy of from cell to module to system. Systems are improving. However, specific energy is improving more than energy density. -
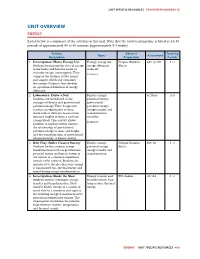
Unit Overview Energy
UNIT SPECIFIC RESOURCES TEACHER RESOURCES IV UNIT OVERVIEW ENERGY Listed below is a summary of the activities in this unit. Note that the total teaching time is listed as 23-35 periods of approximately 45 to 50 minutes (approximately 5-7 weeks). Activity Advance Teaching Topics Assessment Description Preparation Periods 1. Investigation: Home Energy Use Energy, energy use, Prepare Student E&T QC A5 1–2 Students brainstorm the uses of energy energy effciency, Sheets. in the home and become aware of trade-off everyday energy consumption. They LITERACY compare the features of two homes and suggest which one consumes less energy. Students then develop an operational defnition of energy effciency. 2. Laboratory: Drive a Nail Kinetic energy, PCI Proc. 2–3 Students are introduced to the potential energy, concepts of kinetic and gravitational gravitational potential energy. They design and potential energy, conduct an experiment to drop energy transfer and metal rods of different masses from transformation, different heights to drive a nail into variables a foam block. This activity allows LITERACY students to explore energy transfer, the relationship of gravitational potential energy to mass and height, and the transformation of gravitational potential energy to kinetic energy. 3. Role Play: Roller Coaster Energy Kinetic energy, Prepare Student EXP: A1 1–2 Students further examine energy potential energy, Sheet. transformations between gravitational energy transfer and potential energy and kinetic energy in transformation the context of a common experience— namely, roller coasters. Students are introduced to the idea that some energy is transformed into thermal energy and sound during energy transformations. 4. -

Cryometric Determination of the Enthalpy of Fusion of Sodium Cryolite
Cryometric determination of the enthalpy of fusion of sodium cryolite M. MALINOVSKÝ Department of Chemical Technology of Inorganic Substances, Slovak Technical University, CS-812 37Bratislava Received 30 July 1982 Accepted for publication 22 August 1983 Using the method of classical thermal analysis a part of the liquidus of sodium cryolite in the system Na3AlF6—Na3FS04 was measured in the compo sition range 0—5 mole % Na3FS04. On the basis of these results the molar enthalpy of fusion of cryolite was calculated. It was found that AHf(Na3AlFft) = = 115.4 kJ mol"1; error in this result was estimated to be ±4.9 %. Методом классического термического анализа измерена часть ликви дуса натриевого криолита в системе Na3AlF6—Na3FS04 в интервале кон центраций 0—5 мол. % Na3FS04. На основании этих результатов рассчи тана мольная энтальпия плавления криолита. Найдено, что 1 AH,(Na3AlF6) = 115,4 кДж моль" ; точность этого результата ±4,9 %. Literature survey and theoretical Knowledge of the enthalpy of fusion of sodium hexafluoroaluminate Na3AlF6 (cryolite) is important both from theoretical and practical points of view. For example, it is needed at calculation of the thermal and energy balance and/or the thermal inertia of aluminium cells (the electrolyte contains about 90 mass % of Na3AlF6). The most precise values of the molar enthalpy of fusion AHf(i) of chemical substances can be generally obtained from calorimetric measurements. However, when we deal with substances having higher melting temperature and which are also unstable and very corrosive the calorimetric determination of the quantity AHf(i) can be less reliable. In the case of cryolite Roth and Bertram [1] found from the calorimetric 1 1 measurements ÄH,(Na3 A1F6) = 16.64 kcal mol" = 69.62 kJ mol" . -

Energy and Temperature Analysis in Grinding
Transactions on Engineering Sciences vol 44, © 2003 WIT Press, www.witpress.com, ISSN 1743-3533 Energy and temperature analysis in grinding W.B. Rowel, M.N. Morganl, A. Batakol & T. Jin2 I School of Engineering, Liverpool John Moores University, UK 2 S.I.MS. CranJield University, UK Abstract Energy consumption and dissipation are discussed, leading into a thermal model for grinding. The analysis developed over many years applies to shallow-cut conventional grinding processes and also to deep grinding processes. Energy analysis provides insights into the grinding process and suggests avenues for process improvements. The thermal model provides a good estimation of contact temperatures as well as temperatures on the finish surface. Case studies are presented to demonstrate how operational efficiency, component quality and removal rates are affected by process conditions. Examples are included for High Efficiency Deep Grinding (HEDG). HEDG is defined as deep grinding at high workspeeds and very high removal rates. Tawakoli [20], Klocke [21]. The contact between the workpiece and wheel is represented as a circular arc. Experiments show that high removal rates and absence of thermal damage can be achieved. HEDG can achieve low specific grinding energy compared with shallow grinding and creep grinding. The chips take away most of the heat generated in the grinding process. As in creep grinding, bum-out of the coolant causes a steep rise in contact temperature of the workpiece. 1 Introduction Cost, quality and productivity in grinding processes are all dependent on energy consumption and process efficiency. In an inefficient process, excessive heat leads to increased temperature rise in the workpiece causing problems with surface integrity and require more frequent interruptions of production.