Clinical Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Looking for New Classes of Bronchodilators
REVIEW BRONCHODILATORS The future of bronchodilation: looking for new classes of bronchodilators Mario Cazzola1, Paola Rogliani 1 and Maria Gabriella Matera2 Affiliations: 1Dept of Experimental Medicine, University of Rome Tor Vergata, Rome, Italy. 2Dept of Experimental Medicine, University of Campania “Luigi Vanvitelli”, Naples, Italy. Correspondence: Mario Cazzola, Dept of Experimental Medicine, University of Rome Tor Vergata, Via Montpellier 1, Rome, 00133, Italy. E-mail: [email protected] @ERSpublications There is a real interest among researchers and the pharmaceutical industry in developing novel bronchodilators. There are several new opportunities; however, they are mostly in a preclinical phase. They could better optimise bronchodilation. http://bit.ly/2lW1q39 Cite this article as: Cazzola M, Rogliani P, Matera MG. The future of bronchodilation: looking for new classes of bronchodilators. Eur Respir Rev 2019; 28: 190095 [https://doi.org/10.1183/16000617.0095-2019]. ABSTRACT Available bronchodilators can satisfy many of the needs of patients suffering from airway disorders, but they often do not relieve symptoms and their long-term use raises safety concerns. Therefore, there is interest in developing new classes that could help to overcome the limits that characterise the existing classes. At least nine potential new classes of bronchodilators have been identified: 1) selective phosphodiesterase inhibitors; 2) bitter-taste receptor agonists; 3) E-prostanoid receptor 4 agonists; 4) Rho kinase inhibitors; 5) calcilytics; 6) agonists of peroxisome proliferator-activated receptor-γ; 7) agonists of relaxin receptor 1; 8) soluble guanylyl cyclase activators; and 9) pepducins. They are under consideration, but they are mostly in a preclinical phase and, consequently, we still do not know which classes will actually be developed for clinical use and whether it will be proven that a possible clinical benefit outweighs the impact of any adverse effect. -

Human Erythrocyte Acetylcholinesterase in Health and Disease
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Universidade de Lisboa: Repositório.UL molecules Review Human Erythrocyte Acetylcholinesterase in Health and Disease Carlota Saldanha Instituto de Bioquímica, Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Av. Professor Egas Moniz, 1649-028 Lisboa, Portugal; [email protected] Received: 10 August 2017; Accepted: 4 September 2017; Published: 8 September 2017 Abstract: The biochemical properties of erythrocyte or human red blood cell (RBC) membrane acetylcholinesterase (AChE) and its applications on laboratory class and on research are reviewed. Evidence of the biochemical and the pathophysiological properties like the association between the RBC AChE enzyme activity and the clinical and biophysical parameters implicated in several diseases are overviewed, and the achievement of RBC AChE as a biomarker and as a prognostic factor are presented. Beyond its function as an enzyme, a special focus is highlighted in this review for a new function of the RBC AChE, namely a component of the signal transduction pathway of nitric oxide. Keywords: acetylcholinesterase; red blood cells; nitric oxide 1. Introduction Erythrocytes or red blood cells (RBC) are more than sacks of oxyhemoglobin or deoxyhemoglobin during the semi-life of 120 days in blood circulation [1]. Erythrocytes comport different signaling pathways which includes the final stage of apoptosis, also called eryptosis [2,3]. Exovesicules enriched with acetylcholinesterase (AChE) originated from membranes of aged erythrocytes appear in plasma [4]. Kinetic changes of the AChE enzyme have been observed in old erythrocytes [5]. Previously, AChE in erythrocytes was evidenced as a biomarker of membrane integrity [6]. -

Cyclic Nucleotide Phosphodiesterases in Heart and Vessels
Cyclic nucleotide phosphodiesterases in heart and vessels: A therapeutic perspective Pierre Bobin, Milia Belacel-Ouari, Ibrahim Bedioune, Liang Zhang, Jérôme Leroy, Véronique Leblais, Rodolphe Fischmeister, Grégoire Vandecasteele To cite this version: Pierre Bobin, Milia Belacel-Ouari, Ibrahim Bedioune, Liang Zhang, Jérôme Leroy, et al.. Cyclic nucleotide phosphodiesterases in heart and vessels: A therapeutic perspective. Archives of cardiovascular diseases, Elsevier/French Society of Cardiology, 2016, 109 (6-7), pp.431-443. 10.1016/j.acvd.2016.02.004. hal-02482730 HAL Id: hal-02482730 https://hal.archives-ouvertes.fr/hal-02482730 Submitted on 23 Mar 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Cyclic nucleotide phosphodiesterases in heart and vessels: A therapeutic perspective Abbreviated title: Cyclic nucleotide phosphodiesterases in heart and vessels French title: Phosphodiestérases des nucléotides cycliques dans le cœur et les vaisseaux : une perspective thérapeutique. Pierre Bobin, Milia Belacel-Ouari, Ibrahim Bedioune, Liang Zhang, Jérôme Leroy, Véronique Leblais, Rodolphe Fischmeister*, Grégoire Vandecasteele* UMR-S 1180, INSERM, Université Paris-Sud, Université Paris-Saclay, Châtenay-Malabry, France * Corresponding authors. UMR-S1180, Faculté de Pharmacie, Université Paris-Sud, 5 rue J.-B. Clément, F-92296 Châtenay-Malabry Cedex, France. -

Interaction of Phosphodiesterase 3A with Brefeldin A-Inhibited Guanine Nucleotide-Exchange Proteins BIG1 and BIG2 and Effect on ARF1 Activity
Interaction of phosphodiesterase 3A with brefeldin A-inhibited guanine nucleotide-exchange proteins BIG1 and BIG2 and effect on ARF1 activity Ermanno Puxeddu, Marina Uhart, Chun-Chun Li, Faiyaz Ahmad, Gustavo Pacheco-Rodriguez, Vincent C. Manganiello, Joel Moss, and Martha Vaughan1 Translational Medicine Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD 20892 Contributed by Martha Vaughan, February 11, 2009 (sent for review September 22, 2008) ADP-ribosylation factors (ARFs) have crucial roles in vesicular (11). Two-hybrid interaction of the BIG2 N-terminal region with trafficking. Brefeldin A-inhibited guanine nucleotide-exchange exocyst subunit Exo70 was confirmed by coimmunoprecipitation proteins (BIG)1 and BIG2 catalyze the activation of class I ARFs by (co-IP) of the in vitro-translated proteins; microscopically, the accelerating replacement of bound GDP with GTP. Several addi- endogenous proteins appeared together along microtubules tional and differing actions of BIG1 and BIG2 have been described. (12);3Akinase-anchoring protein (AKAP) sequences in the These include the presence in BIG2 of 3 A kinase-anchoring protein N-terminal region of BIG2, one of which is identical in BIG1, (AKAP) domains, one of which is identical in BIG1. Proteins that were identified in yeast 2-hybrid experiments with 4 different A contain AKAP sequences act as scaffolds for the assembly of PKA kinase R subunits and multiple peptide sequences representing with other enzymes, substrates, and regulators in complexes that positions 1 to 643 of BIG2 (13). Consistent with the presence of constitute molecular machines for the reception, transduction, and AKAP sequences for each of the 4 R subunits in BIG1 and BIG2, integration of signals from cAMP or other sources, which are both proteins were coimmunoprecipitated from HepG2 cell initiated, propagated, and transmitted by chemical, electrical, or cytosol with antibodies against RI or RII, although direct mechanical means. -

Erythrocyte Acetylcholinesterase Is a Signaling Receptor
Mini Review Nov Appro Drug Des Dev - Volume 3 Issue 4 January 2018 Copyright © All rights are reserved by Carlota Saldanha DOI: 10.19080/NAPDD.2018.03.555617 Erythrocyte Acetylcholinesterase is a Signaling Receptor Carlota Saldanha* and Ana Silva Herdade Institute of Biochemistry, Institute of Molecular Medicine, Faculty of Medicine, University of Lisbon, Portugal Received Date: January 12, 2018; Published Date: January 23, 2018 *Corresponding author: Carlota Saldanha, Institute of Biochemistry, Institute of Molecular Medicine, Faculty of Medicine, University of Lisbon, Portugal, Email: Abstract The acetylcholinesterase (AChE), is an enzyme located in the erythrocyte membrane that motivate continually questions, about its physiological function. The aim of this mini review is highlight the receptor behavior of AChE in human red blood cells for chemical signals participative proteins in the steps of the signal transduction pathways, in dependence of AChE receptor, are suggested to be further considered asassociated potential with therapeutic nitric oxide targets. mobilization and efflux from erythrocytes. Data from the experimental models used are presented. Consequently, key Keywords: Erythrocyte; Acetylcholinesterase; signaling ; Acetylcholine; Receptor Abbreviations: RBCs: Red Blood Cells; AChE: Acetylcholinesterase; ACh: Acetylcholine; AC: Adenylyl cyclase; cAMP: cyclic Adenosine Monophosphate (cAMP) Mini Review The need to characterize the composition, structure and function of the red blood cells (RBCs) membrane, the presence ALS [10]. These pathologies are inflammatory vascular diseases molecules, reactive oxygen species, and reactive nitrogen species, of the acetylcholinesterase (AChE) enzyme, which kinetically characterized by high concentration in blood of inflammatory [10]. The RBCs AChE enzyme activity data obtained in those resembled the brain esterase, but markedly different from above mentioned vascular diseases reinforce the Das`statement the cholinesterase found in serum was evidenced [1]. -

Supplementary Table 2
Supplementary Table 2. Differentially Expressed Genes following Sham treatment relative to Untreated Controls Fold Change Accession Name Symbol 3 h 12 h NM_013121 CD28 antigen Cd28 12.82 BG665360 FMS-like tyrosine kinase 1 Flt1 9.63 NM_012701 Adrenergic receptor, beta 1 Adrb1 8.24 0.46 U20796 Nuclear receptor subfamily 1, group D, member 2 Nr1d2 7.22 NM_017116 Calpain 2 Capn2 6.41 BE097282 Guanine nucleotide binding protein, alpha 12 Gna12 6.21 NM_053328 Basic helix-loop-helix domain containing, class B2 Bhlhb2 5.79 NM_053831 Guanylate cyclase 2f Gucy2f 5.71 AW251703 Tumor necrosis factor receptor superfamily, member 12a Tnfrsf12a 5.57 NM_021691 Twist homolog 2 (Drosophila) Twist2 5.42 NM_133550 Fc receptor, IgE, low affinity II, alpha polypeptide Fcer2a 4.93 NM_031120 Signal sequence receptor, gamma Ssr3 4.84 NM_053544 Secreted frizzled-related protein 4 Sfrp4 4.73 NM_053910 Pleckstrin homology, Sec7 and coiled/coil domains 1 Pscd1 4.69 BE113233 Suppressor of cytokine signaling 2 Socs2 4.68 NM_053949 Potassium voltage-gated channel, subfamily H (eag- Kcnh2 4.60 related), member 2 NM_017305 Glutamate cysteine ligase, modifier subunit Gclm 4.59 NM_017309 Protein phospatase 3, regulatory subunit B, alpha Ppp3r1 4.54 isoform,type 1 NM_012765 5-hydroxytryptamine (serotonin) receptor 2C Htr2c 4.46 NM_017218 V-erb-b2 erythroblastic leukemia viral oncogene homolog Erbb3 4.42 3 (avian) AW918369 Zinc finger protein 191 Zfp191 4.38 NM_031034 Guanine nucleotide binding protein, alpha 12 Gna12 4.38 NM_017020 Interleukin 6 receptor Il6r 4.37 AJ002942 -

Regulation of Phosphodiesterase 3 in the Pulmonary Arteries During the Perinatal Period in Sheep
0031-3998/09/6606-0682 Vol. 66, No. 6, 2009 PEDIATRIC RESEARCH Printed in U.S.A. Copyright © 2009 International Pediatric Research Foundation, Inc. Regulation of Phosphodiesterase 3 in the Pulmonary Arteries During the Perinatal Period in Sheep BERNADETTE CHEN, SATYAN LAKSHMINRUSIMHA, LYUBOV CZECH, BEEZLY S. GROH, SYLVIA F. GUGINO, JAMES A. RUSSELL, KATHRYN N. FARROW, AND ROBIN H. STEINHORN Department of Pediatrics [B.C., L.C., B.S.G., K.N.F., R.H.S.], Northwestern University, Chicago, Illinois 60611; Departments of Pediatrics [S.L., J.A.R.] and Physiology and Biophysics [S.F.G., J.A.R.], State University of New York, Buffalo, New York 14260 ABSTRACT: The role of cAMP in the pulmonary vasculature predominant isoforms for cAMP degradation. PDE3 has high during the transition from intrauterine to extrauterine life is poorly affinities for both cAMP and cGMP, but the Vmax for cAMP is understood. We hypothesized that cAMP levels are regulated by approximately 4- to 10-fold higher than for cGMP (3,4). As alterations in phosphodiesterase 3 (PDE3), which hydrolyzes cAMP. PDE3 activity is positively regulated by cAMP and negatively PDE3 protein expression and hydrolytic activity were increased in regulated by cGMP (3,5,6), it is referred to as the cGMP- the resistance pulmonary arteries (PA) from spontaneously breathing 1-d-old (1dSB) lambs relative to equivalent-gestation fetuses. This inhibited PDE (Fig. 1). was accompanied by a decrease in steady-state cAMP. Ventilation The AC-cAMP-PDE3 pathway and its role in pulmonary with 21% O2 and 100% O2 for 24 h disrupted the normal transition, vascular smooth muscle relaxation have not been well-studied ϩ whereas ventilation with 100% O2 inhaled NO (iNO) for 24 h perinatally or in infants with impaired transition, such as those restored both PDE3 activity and cAMP to 1dSB levels. -

Various Subtypes of Phosphodiesterase Inhibitors Differentially Regulate Pulmonary Vein and Sinoatrial Node Electrical Activities
EXPERIMENTAL AND THERAPEUTIC MEDICINE 19: 2773-2782, 2020 Various subtypes of phosphodiesterase inhibitors differentially regulate pulmonary vein and sinoatrial node electrical activities YUNG‑KUO LIN1,2*, CHEN‑CHUAN CHENG3*, JEN‑HUNG HUANG1,2, YI-ANN CHEN4, YEN-YU LU5, YAO‑CHANG CHEN6, SHIH‑ANN CHEN7 and YI-JEN CHEN1,8 1Department of Internal Medicine, Division of Cardiovascular Medicine, Wan Fang Hospital; 2Department of Internal Medicine, Division of Cardiology, School of Medicine, College of Medicine, Taipei Medical University, Taipei 11696; 3Division of Cardiology, Chi‑Mei Medical Center, Tainan 71004; 4Division of Nephrology; 5Division of Cardiology, Department of Internal Medicine, Sijhih Cathay General Hospital, New Taipei 22174; 6Department of Biomedical Engineering, National Defense Medical Center, Taipei 11490; 7Heart Rhythm Center and Division of Cardiology, Department of Medicine, Taipei Veterans General Hospital, Taipei 11217; 8Graduate Institute of Clinical Medicine, College of Medicine, Taipei Medical University, Taipei 11696, Taiwan, R.O.C. Received May 16, 2019; Accepted January 9, 2020 DOI: 10.3892/etm.2020.8495 Abstract. Phosphodiesterase (PDE)3-5 are expressed in 20.7±4.6%, respectively. In addition, milrinone (1 and 10 µM) cardiac tissue and play critical roles in the pathogenesis of induced the occurrence of triggered activity (0/8 vs. 5/8; heart failure and atrial fibrillation. PDE inhibitors are widely P<0.005) in PVs. Rolipram increased PV spontaneous activity used in the clinic, but their effects on the electrical activity by 7.5±1.3‑9.5±4.0%, although this was not significant, and did of the heart are not well understood. The aim of the present not alter SAN spontaneous activity. -

Table SII. Significantly Differentially Expressed Mrnas of GSE23558 Data Series with the Criteria of Adjusted P<0.05 And
Table SII. Significantly differentially expressed mRNAs of GSE23558 data series with the criteria of adjusted P<0.05 and logFC>1.5. Probe ID Adjusted P-value logFC Gene symbol Gene title A_23_P157793 1.52x10-5 6.91 CA9 carbonic anhydrase 9 A_23_P161698 1.14x10-4 5.86 MMP3 matrix metallopeptidase 3 A_23_P25150 1.49x10-9 5.67 HOXC9 homeobox C9 A_23_P13094 3.26x10-4 5.56 MMP10 matrix metallopeptidase 10 A_23_P48570 2.36x10-5 5.48 DHRS2 dehydrogenase A_23_P125278 3.03x10-3 5.40 CXCL11 C-X-C motif chemokine ligand 11 A_23_P321501 1.63x10-5 5.38 DHRS2 dehydrogenase A_23_P431388 2.27x10-6 5.33 SPOCD1 SPOC domain containing 1 A_24_P20607 5.13x10-4 5.32 CXCL11 C-X-C motif chemokine ligand 11 A_24_P11061 3.70x10-3 5.30 CSAG1 chondrosarcoma associated gene 1 A_23_P87700 1.03x10-4 5.25 MFAP5 microfibrillar associated protein 5 A_23_P150979 1.81x10-2 5.25 MUCL1 mucin like 1 A_23_P1691 2.71x10-8 5.12 MMP1 matrix metallopeptidase 1 A_23_P350005 2.53x10-4 5.12 TRIML2 tripartite motif family like 2 A_24_P303091 1.23x10-3 4.99 CXCL10 C-X-C motif chemokine ligand 10 A_24_P923612 1.60x10-5 4.95 PTHLH parathyroid hormone like hormone A_23_P7313 6.03x10-5 4.94 SPP1 secreted phosphoprotein 1 A_23_P122924 2.45x10-8 4.93 INHBA inhibin A subunit A_32_P155460 6.56x10-3 4.91 PICSAR P38 inhibited cutaneous squamous cell carcinoma associated lincRNA A_24_P686965 8.75x10-7 4.82 SH2D5 SH2 domain containing 5 A_23_P105475 7.74x10-3 4.70 SLCO1B3 solute carrier organic anion transporter family member 1B3 A_24_P85099 4.82x10-5 4.67 HMGA2 high mobility group AT-hook 2 A_24_P101651 -

The Metabolic Building Blocks of a Minimal Cell Supplementary
The metabolic building blocks of a minimal cell Mariana Reyes-Prieto, Rosario Gil, Mercè Llabrés, Pere Palmer and Andrés Moya Supplementary material. Table S1. List of enzymes and reactions modified from Gabaldon et. al. (2007). n.i.: non identified. E.C. Name Reaction Gil et. al. 2004 Glass et. al. 2006 number 2.7.1.69 phosphotransferase system glc + pep → g6p + pyr PTS MG041, 069, 429 5.3.1.9 glucose-6-phosphate isomerase g6p ↔ f6p PGI MG111 2.7.1.11 6-phosphofructokinase f6p + atp → fbp + adp PFK MG215 4.1.2.13 fructose-1,6-bisphosphate aldolase fbp ↔ gdp + dhp FBA MG023 5.3.1.1 triose-phosphate isomerase gdp ↔ dhp TPI MG431 glyceraldehyde-3-phosphate gdp + nad + p ↔ bpg + 1.2.1.12 GAP MG301 dehydrogenase nadh 2.7.2.3 phosphoglycerate kinase bpg + adp ↔ 3pg + atp PGK MG300 5.4.2.1 phosphoglycerate mutase 3pg ↔ 2pg GPM MG430 4.2.1.11 enolase 2pg ↔ pep ENO MG407 2.7.1.40 pyruvate kinase pep + adp → pyr + atp PYK MG216 1.1.1.27 lactate dehydrogenase pyr + nadh ↔ lac + nad LDH MG460 1.1.1.94 sn-glycerol-3-phosphate dehydrogenase dhp + nadh → g3p + nad GPS n.i. 2.3.1.15 sn-glycerol-3-phosphate acyltransferase g3p + pal → mag PLSb n.i. 2.3.1.51 1-acyl-sn-glycerol-3-phosphate mag + pal → dag PLSc MG212 acyltransferase 2.7.7.41 phosphatidate cytidyltransferase dag + ctp → cdp-dag + pp CDS MG437 cdp-dag + ser → pser + 2.7.8.8 phosphatidylserine synthase PSS n.i. cmp 4.1.1.65 phosphatidylserine decarboxylase pser → peta PSD n.i. -
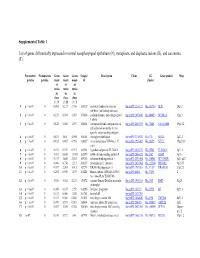
Supplemental Table 1 List of Genes Differentially Expressed In
Supplemental Table 1 List of genes differentially expressed in normal nasopharyngeal epithelium (N), metaplastic and displastic lesions (R), and carcinoma (T). Parametric Permutation Geom Geom Geom Unique Description Clone UG Gene symbol Map p-value p-value mean mean mean id cluster of of of ratios ratios ratios in in in class class class 1 : N 2 : R 3 : T 1 p < 1e-07 0 0.061 0.123 2.708 169329 secretory leukocyte protease IncytePD:2510171 Hs.251754 SLPI 20q12 inhibitor (antileukoproteinase) 2 p < 1e-07 0 0.125 0.394 1.863 163628 sodium channel, nonvoltage-gated IncytePD:1453049 Hs.446415 SCNN1A 12p13 1 alpha 3 p < 1e-07 0 0.122 0.046 1.497 160401 carcinoembryonic antigen-related IncytePD:2060355 Hs.73848 CEACAM6 19q13.2 cell adhesion molecule 6 (non- specific cross reacting antigen) 4 p < 1e-07 0 0.675 1.64 5.594 165101 monoglyceride lipase IncytePD:2174920 Hs.6721 MGLL 3q21.3 5 p < 1e-07 0 0.182 0.487 0.998 166827 nei endonuclease VIII-like 1 (E. IncytePD:1926409 Hs.28355 NEIL1 15q22.33 coli) 6 p < 1e-07 0 0.194 0.339 0.915 162931 hypothetical protein FLJ22418 IncytePD:2816379 Hs.36563 FLJ22418 1p11.1 7 p < 1e-07 0 1.313 0.645 13.593 162399 S100 calcium binding protein P IncytePD:2060823 Hs.2962 S100P 4p16 8 p < 1e-07 0 0.157 1.445 2.563 169315 selenium binding protein 1 IncytePD:2591494 Hs.334841 SELENBP1 1q21-q22 9 p < 1e-07 0 0.046 0.738 1.213 160115 prominin-like 1 (mouse) IncytePD:2070568 Hs.112360 PROML1 4p15.33 10 p < 1e-07 0 0.787 2.264 3.013 167294 HRAS-like suppressor 3 IncytePD:1969263 Hs.37189 HRASLS3 11q12.3 11 p < 1e-07 0 0.292 0.539 1.493 168221 Homo sapiens cDNA FLJ13510 IncytePD:64451 Hs.37896 2 fis, clone PLACE1005146. -

Dual Activation of Phosphodiesterase 3 and 4 Regulates Basal Cardiac Pacemaker Function and Beyond
International Journal of Molecular Sciences Review Dual Activation of Phosphodiesterase 3 and 4 Regulates Basal Cardiac Pacemaker Function and Beyond Tatiana M. Vinogradova * and Edward G. Lakatta Laboratory of Cardiovascular Science, Intramural Research Program, National Institute on Aging, National Institute of Health, 251 Bayview Boulevard, Baltimore, MD 21224, USA; [email protected] * Correspondence: [email protected] Abstract: The sinoatrial (SA) node is the physiological pacemaker of the heart, and resting heart rate in humans is a well-known risk factor for cardiovascular disease and mortality. Consequently, the mechanisms of initiating and regulating the normal spontaneous SA node beating rate are of vital importance. Spontaneous firing of the SA node is generated within sinoatrial nodal cells (SANC), which is regulated by the coupled-clock pacemaker system. Normal spontaneous beating of SANC is driven by a high level of cAMP-mediated PKA-dependent protein phosphorylation, which rely on the balance between high basal cAMP production by adenylyl cyclases and high basal cAMP degradation by cyclic nucleotide phosphodiesterases (PDEs). This diverse class of enzymes includes 11 families and PDE3 and PDE4 families dominate in both the SA node and cardiac myocardium, degrading cAMP and, consequently, regulating basal cardiac pacemaker function and excitation-contraction coupling. In this review, we will demonstrate similarities between expression, distribution, and colocalization of various PDE subtypes in SANC and cardiac myocytes of different species, including humans, focusing on PDE3 and PDE4. Here, we will describe specific targets of the coupled-clock pacemaker system modulated by dual PDE3 + PDE4 activation and provide Citation: Vinogradova, T.M.; Lakatta, evidence that concurrent activation of PDE3 + PDE4, operating in a synergistic manner, regulates the E.G.