Internal Audit Team, Coordinating Their Activities and Thus Achieving Significantly Greater Operational Efficiency
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Supplementary Protection Mechanisms for Pharmaceutical Products
Final report, May 2018 Effects of supplementary protection mechanisms for pharmaceutical products www.technopolis-group.com Effects of supplementary protection mechanisms for pharmaceutical products technopolis |group| May 2018 Thyra de Jongh* Alfred Radauer Sven Bostyn Joost Poort * Corresponding author. [email protected] About the authors Thyra de Jongh PhD, is a Senior Consultant Health & Life Sciences at Technopolis Group in Amsterdam. She specialises in analysis of health systems and policies, and research and innovation in the health and life sciences. She has a particular interest in pharmaceutical innovation and access to medicine. (thyra.dejongh@technopolis- group.com) Alfred Radauer, is Senior Consultant at Technopolis Group in Vienna and leads the Intellectual Property & Standards (IPS) group within Technopolis. His and the IPS group work elaborate on the interfaces between legal, technological and economic issues of IP and standards in innovation policy as well as the interaction of IP with regulation and other policy areas. ([email protected]) Sven Bostyn Lic.Jur, LL.M, PhD, is a senior lecturer in Intellectual Property Law at the University of Liverpool Law School. He is also an assistant professor at the Institute for Information Law, University of Amsterdam. He is one of the leading experts in Europe in patent law and related subjects in the areas of life sciences and pharmaceutical inventions, and has more than sixty single-authored publications. ([email protected]) Joost Poort PhD, is an associate professor in economics at the Institute for Information law, University of Amsterdam. He brings an economic perspective to various multidisciplinary research projects, with particular interest in intellectual property. -

Action Plan for Vidzeme Region
Cult ural Routes as Investments for Growth and Jobs Action Plan for Vidzeme Region December 2018 Cultural Routes as Investment for Growth and Jobs _________________________ © Cult-RInG Project Partnership and Vidzeme Tourism Association, Latvia This publication may be reproduced in whole or in part and in any form for educational or non-profit purposes without special permission from the copyright holder, provided acknowledgement of the source is made. No use of this publication may be made for resale or for any other commercial purpose whatsoever without prior permission in writing from the Cult-RInG Project Communication and the respective partner: Vidzeme Tourism Association. Citation: Interreg Europe Project Cult-RInG Action Plan, Vidzeme Tourism Association, Latvia Cult-RInG project communication unit would appreciate receiving a copy of any publication that uses this publication as a source on e-mail: [email protected] Disclaimer This document has been prepared with the financial support of Interreg Europe 2014-2020 interregional cooperation programme. The content of the document is the sole responsibility of Vidzeme Tourism Association and in no way reflect the views of the European Union institutions, nor the Managing Authority of the Programme. Any reliance or action taken based on the information, materials and techniques described within this document are the responsibility of the user. The Cult-RInG project partnership assumes no responsibility for any consequences arising from use of the information described within this document. December 2018 Action Plan 2 Cultural Routes as Investment for Growth and Jobs Interreg Europe Programme Interreg Europe Programme of interregional cooperation helps regional and local governments across Europe to develop and deliver better policy. -

List of Tuberculosis Pharmaceutical Products Classified According to the Global Fund Quality Assurance Policy
List Of Tuberculosis Pharmaceutical Products classified according to the Global Fund Quality Assurance Policy Edition: Version 104 - 14 July 2021 The list is an overview of pharmaceutical products subject to the Global Fund Quality Assurance Policy that are listed in National and/or WHO standard treatment guidelines and classified according to the various options (A, B, and ERP reviewed) defined in the Global Fund Quality Assurance Policy (July 2009, amended in December 2010). The list is developed as a tool to assist Principal Recipients (PR) of Global Fund grants to identify the status of finished pharmaceutical products according to the Global Fund Quality Assurance Policy. The pharmaceutical products are classified based on the following information A classified product - Listed on WHO prequalification list; B classified product - Stringent NDRA Registration letter/Marketing Authorization; ERP reviewed product- Permitted for time-limited procurement based on advice by the Expert Review Panel (ERP); Please note that the list is not an exhaustive list. A PR can procure product(s) not listed in the current list as long as PR demonstrates that the product is compliant with the Global Fund Quality Assurance Policy. The Global Fund list includes the following information: - "Important Notes" for helpful information; - A, B, and ERP reviewed products; - Period validity of the ERP reviewed products; - "+" means combination product, both fixed-dose combination (co-formulated) and co-packaged product ( i.e. co-blister) - [A+B]+C means A and B are in fixed-dose formulation and C is co-packaged YELLOW signifies a new entry; PINK signifies modification of an existing entry. -

Points of Excellence
POINTS OF EXCELLENCE www.rtu.lv Riga Technical University, 2016 Published by: International Relations Department Design and layout: Arnis Grinbergs Photo credits: Arturs Pērkons, Eduards Lapsa, Edgars Špengelis, Aleksandrs Kendenkovs, Andris Eglītis, Jānis Knāķis, Edijs Pālēns / LETA, Imants Urtāns, Valts Kleins POINTS OF EXCELLENCE 6 Riga Technical University (RTU) – Strategic Partner for Excellence 72 Riga Business School 8 Latvia and Riga – “Added Value” of RTU 73 BALTECH Study Center 10 The Republic of Latvia 74 Affiliations 14 The Capital City – Riga 75 Engineering High School 20 History of RTU 76 Research at RTU 28 RTU in the 21st Century 77 Research Management 30 RTU Ķīpsala Campus 78 Research platforms and main research areas 36 RTU Structure 80 Selected Research Projects 39 RTU Strategy 94 Cooperation with Enterprises 40 Studies at RTU 95 Start-Up and Spin-Off Enterprises 40 Study Process Management 96 Business Incubators 42 Faculty of Architecture and Urban Planning 98 Design Factory 46 Faculty of Civil Engineering 100 Scientific Library 48 Faculty of Computer Science and Information Technology 104 Student Parliament 50 Faculty of Electronics and Telecommunications 106 International Relations CONTENTS 54 Faculty of Power and Electrical Engineering 116 Sports 58 Faculty of E-Learning Technologies and Humanities 120 Culture 60 Faculty of Engineering Economics and Management 128 RTU Alumni Association 64 Faculty of Materials Science and Applied Chemistry 136 RTU Contacts 68 Faculty of Mechanical Engineering, Transport and Aeronautics niversity should not just react at Despite a marked focus on engineering, we pay changes in the society; it should be the much attention to sports and cultural activities – driving force for change! Riga Technical RTU teams in basketball, volleyball and floorball University adheres to this principle in have won medals in both Latvian and European Uthe field of tertiary education and is constantly championships. -

ESG Report 2018
JSC Olainfarm ENVIRONMENTAL, SOCIAL AND GOVERNANCE REPORT 2018 Report is based on Guidelines of NASDAQ ESG Guide for Nordic and Baltic Markets ENVIRONMENTAL, SOCIAL AND GOVERNANCE REPORT 2018 Start of financial year: 1 January 2018 End of financial year: 31 December 2018 Company name: Joint Stock Company Olainfarm Unified registration No. 40003007246 Address and contacts: Olaine Municipality, Olaine, 5 Rupnicu Street, LV-2114, Latvia (+371) 67013705 E-mail: [email protected] Chairman of the Board: Lauris Macijevskis Area of business: Pharmacology Website: www.olainfarm.com Auditing company: LLC PricewaterhouseCoopers Shares of JSC Olainfarm are listed at Nasdaq Riga Ocial List (ISIN:LV0000100501, ID: OLF1R) ENVIRONMENTAL, SOCIAL AND GOVERNANCE REPORT 2018 Table of Contents 5 15 37 Company Environmental Quality management • Mission, Vision and Main Tasks 5 Responsibility • Shareholders 7 • Finished Dosage Forms, Active • Environmental Policy and Regulating Pharmaceutical Ingredients and Documents 16 40 Intermediates 7 • Control of Greenhouse Gas Emissions 17 • Largest Subsidiaries 7 Corporate governance • Employees 8 • Management of Environmental Risks 18 • Representation of Interests 8 • Monitoring of Environmental Risks 19 • Responsible Business Environment and • Packaging Policy 25 Cooperation with NGOs 41 • Waste Management 25 • Responsible Board 41 • Recycled Materials 28 - Obligations and responsibility 9 of the Board - Composition of the Board and Chairman’s 29 Requirements to Members of the Board - Transparent work of the board -

PHARMACEUTICALS + Biovian Oy Jemedic AB Bayer Oy + Shetland Bergen Helse Bergen HF, Haukeland 3
BARENTS SEA Jan Mayen (Norway) Tromsø NORWAY Murmansk DENMARK STRAIT NORWEGIAN SEA Raufarhöfn Bolungarvik Kalix Aromtech Oy Mosjøen SWEDEN Kemi Akureyri Luleå WHITE SEA Piteå Eskifjördur Oy Woikoski Ab + Pharmatory Ltd + Bioactive Bone Substitutes Oolu Fermion Oy Olafsvik ICELAND Skellefteå IA Hornafjördur Reykjavik 1-5 Grindavik Apotek Produktion & Laboratorier AB Oy Woikoski Ab Umeå Norrlands Universitetssjukhus Frøya Vik Trondheim Kristiansund GULF OF BOTHN Vaasa Averøya Galena Pharma Oy + Orion Oyj + Finvector Oy Sandøy Härnösand Ålesund Sundsvall Unimedic AB + MAP Medical Technologies Oy Woikoski Ab Kristinestad Tórshavn Apoteksverkiö, Framleiösluapotekiö Måløy EWOS AS Santen Oy Cytomed Oy SWEDEN - Not Shown Vitabalans Oy + Umeå + Curida AS Apotek Produktion & Laboratorier AB Oy Aga Ab Norrlands Universitetssjukhus Jemedic AB FINLAND OSLO Gävle Alby Vyborg + Nouryon Pulp & Performance Chemicals AB Hankintatukku Oy 1. Smerud Medical Research Norway AS Pcas Finland Oy Finex Oy Hamina Orion Corporation Oy Woikoski Ab 2. Catapult Life Science AS Gävle + + EUROPE - PHARMACEUTICALS + Biovian Oy Jemedic AB Bayer Oy + Shetland Bergen Helse Bergen HF, Haukeland 3. PhotoCure ASA Turku Orion Corporation A.Vogel Oy universitetssjukehus 4. GE Healthcare AS Vitabalans Oy MAP Medicals Technologies Oy + Nanoform Finland Oy Islands 5. Oncoinvent AS Fermion Oy AGA Gas AB Lumene Oy Lerwick 6. The Cyclotron Center Pharmaq AS + Scan Aqua AS Orion Corporation + 7. Oslo Universitetssykehus HF HELSINKI 8. Diatec Monoclonals AS Institute for Energy Technology -

Copyright Notice
www.generics-bulletin.com Bulletin Publishing Group 4 Poplar Road, Dorridge, Solihull B93 8DB, UK Tel: +44 (0)1564 777550 Fax: +44 (0)1564 777524 E-mail: [email protected] COPYRIGHT NOTICE This publication must not be forwarded, exported, distributed or circulated by any means or in any format to persons including clients outside the direct employment of your Company. You may distribute the publication internally, but you may incorporate only insubstantial extracts, abstractsbulletin or summaries into presentations, providing Generics is identified as the source of the information. bulletin bulletin The publication/s, Generics and/or News@Generics , in PDF and/or HTML format, are supplied to you strictly under the terms and conditions of the Global Licence agreement between your Company and OTC Publications Ltd, the copyright holder of the publications. The publication/s are the intellectual property of the Publisher, OTC Publications Ltd and are protected by English copyright, trademark and other laws. Bulletin Publishing Group is a division of OTC Publications Ltd Registered Office: 4 Poplar Road, Dorridge, Solihull B93 8DB, UK. Registered in England No 2765878 Gen 18/4/14 Pg. 1_Gen 18/11/05 Pg. 1 15/04/2014 20:12 Page 1 18 April 2014 COMPANY NEWS 2 Sun makes US$4 billion Mylan misses out on 2 move to gain Meda Pamplona fund takes control of Alvogen 3 Bioton benefits as sales 4 bid to snap up Ranbaxy of insulin boom Sun seeks synergies from Ranbaxy deal 5 un Pharmaceutical Industries intends to create the world’s fifth-largest generics player, Latvia’sOlainfarm sees 6 Sand India’s leading pharma company,bypaying around US$4.0 billion forits rival, upside to crisis Ranbaxy.The deal comprises US$3.2 billion of equity,and US$800 million of assumed debt. -

Audited Country Address Product Type Audit Date Audit Format
AZBIL TELSTAR TECHNOLOGIES, S.L.U. www.telstar-lifesciences.com Tel/Fax: +34 937 361 600 / +34 937 861 380 Audited Country Address Product type Audit Date Audit Format SOLARA ACTIVE PHARMA R. S. No. 33 & 34, Mathur Road, SCIENCES LIMITED (antes India Periyakalapet, Puducherry, IN- API 4-Jan-2019 1d/1a+CAPA STRIDES SHASUN LIMITED) 605 014 India PLOT NO. E-22 M.I.D.C. Tarapur, AARTI DRUGS LTD. India Tal. Palghar, District Thane, 401 API 3-Jul-2019 1d/1a+CAPA 506, Tarapur, Maharashtra. India SOLARA ACTIVE PHARMA R. S. No. 33 & 34, Mathur Road, SCIENCES LIMITED (antes India Periyakalapet, Puducherry, IN- API 4-Jan-2019 1d/1a+CAPA STRIDES SHASUN LIMITED) 605 014 India PLOT NO. E-22 M.I.D.C. Tarapur, AARTI DRUGS LTD. India Tal. Palghar, District Thane, 401 API 3-Jul-2019 1d/1a+CAPA 506, Tarapur, Maharashtra. India Carrer Radi, 08755 Castellbisbal, OLMED SPAIN OTROS 10-May-2017 1d/1a+CAPA Barcelona Plot No. W-60(B), 61 (B), 62(B), 71 (B), 72(B), 73(B), M.I.D.C. AARTI DRUGS LTD. India API 5-Jul-2019 1d/1a+CAPA Taranpur, Boisar, Dist. Thane, Maharashtra 401506 INDIA Audit Reports_January 2020 AZBIL TELSTAR TECHNOLOGIES, S.L.U. www.telstar-lifesciences.com Tel/Fax: +34 937 361 600 / +34 937 861 380 Plot No. W 71(B) & 72(B) M.I.D.C. Tarapur, Tal. Palghar, District AARTI DRUGS LTD. India API 20-Jun-2018 1d/1a+CAPA Thane, India-401 506 Tarapur, Maharashtra - India Plot No. W 71(B) & 72(B) M.I.D.C. -

Analysis of the Global Fund Financed Market for TB Medicines: Competitive Landscape, Market Shares, Product Volumes and Product Variety
Fall 08 2014 Analysis of the Global Fund Financed Market for TB Medicines: Competitive landscape, market shares, product volumes and product variety Lisa Smith & Prashant Yadav January 2014 1 Acknowledgements This analysis was possible mainly due to the publicly available transaction data in the Price, Quality and Reporting database of the Global Fund to Fight AIDS, Tuberculosis & Malaria. The authors would like to thank all associated with conceptualizing, developing, designing and improving this database. In addition, the authors would like to acknowledge support from UNITAID to understand the market for TB drugs. Disclaimer All errors of fact, logic, assumption, and judgment are only those of the authors and should not be attributed to the sources of data or the institutions or individuals listed earlier. The analysis and views expressed in this report are those of the authors and do not necessarily reflect those of the management or the funders of the William Davidson Institute. 2 Table of Contents BACKGROUND 4 PRODUCT MARKET ANALYSIS 7 FIRST-LINE DRUGS 7 ETHAMBUTOL + ISONIAZID – FIXED DOSE COMBINATION 7 ETHAMBUTOL + ISONIAZID + PYRAZINAMIDE + RIFAMPICIN – FIXED DOSE COMBINATION 7 ETHAMBUTOL + ISONIAZID + RIFAMPICIN – FIXED DOSE COMBINATION 11 ISONIAZID + PYRAZINAMIDE + RIFAMPICIN – FIXED DOSE COMBINATION, DISPERSIBLE 13 ISONIAZID + RIFAMPICIN 16 SECOND-LINE DRUGS 25 INJECTABLES AGENTS 25 AMIKACIN 25 CAPREOMYCIN 26 KANAMYCIN 29 STREPTOMYCIN 32 FLUOROQUINOLONES 35 LEVOFLOXACIN 35 MOXIFLOXACIN 38 ORAL BACTERIOSTATIC SECOND-LINE AGENTS 41 CYCLOSERINE 41 PARA-AMINOSALICYLIC ACID (PAS) AND PAS SODIUM 43 PROTHIONAMIDE 47 TUBERCULOSIS TREATMENT KITS 48 SINGLE SUPPLY MARKETS 50 SINGLE SUPPLIER PRODUCT MARKETS – 2011 50 SINGLE SUPPLIER PRODUCT MARKETS – 2012 51 SINGLE SUPPLIER PRODUCT MARKETS – 2011/2012 52 3 Background Ensuring consistent access to affordable, quality tuberculosis (TB) medications remains a key focus area in global health. -
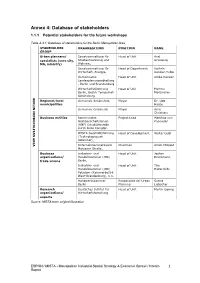
Database of Stakeholders 1.1.1 Potential Stakeholders for the Future Workshops
Annex 4: Database of stakeholders 1.1.1 Potential stakeholders for the future workshops Table A.4.1: Database of stakeholders for the Berlin Metropolitan Area STAKEHOLDER ORGANIZATION POSITION NAME GROUP Urban planners/ Senatsverwaltung für Head of Unit Axel specialists (core city, Stadtentwicklung und Grünberg MA, ministry) Wohnen, Senatsverwaltung für Head of Department Kathrin Wirtschaft, Energie, Kanzler-Tullio Gemeinsame Head of Unit Ulrike Kessler Landesplanungsabteilung , Berlin und Brandenburg Wirtschaftsförderung Head of Unit Martina Berlin, Bezirk Tempelhof- Marijnissen Schöneberg BERLIN METROPOLITAN AREA METROPOLITAN BERLIN Regional/local Gemeinde Schönefeld, Mayor Dr. Udo municipalities Haase Gemeinde Grünheide Mayor Arne Christiani Business entities Kommunales Project Lead Matthias von Nachbarschaftsforum Popowski (KNF) Geschäftsstelle durch Büro Complan WISTA Geschäftsführung Head of Development Walter Leibl (Technologiepark Adlershof), Unternehmensnetzwerk Chairman Ulrich Misgeld Motzener Straße, Business Industrie- und Head of Unit Jochen organisations/ Handelskammer (IHK) Brückmann trade unions Berlin, Industrie- und Head of Unit Tim Handelskammer (IHK) Habernicht Potsdam (Kammerbezirk West-Brandenburg), n.n. Handwerkskammer Responsible for Urban Svend Berlin Planning Liebscher Research Deutsches Institut für Head of Unit Martin Gornig organisations/ Wirtschaftsforschung experts Source: MISTA team original illustration ESPON / MISTA - Metropolitan Industrial Spatial Strategy & Economic Sprawl / Interim 1 Report Table A.4.2: -

Webinar Presentation H1 2021
Webinar Presentation H1 2021 www.olainfarm.com 2 Agenda 01 02 Operational environment Financial performance H1 2021 03 04 Main events Q&A session www.olainfarm.com 3 Today’s speakers Chairman of the Member of the Management Management Board Board www.olainfarm.com 4 Operational Environment www.olainfarm.com Group operations in H1 2021 5 Positive financial results in Extraordinary General Dividends paid on 12 July 6 months Meetings of Shareholders held 2021 in total amount EUR KEY HIGHLIGHTS Key ratios are hitting on 17 June 2021 and 18 June 2 394 thousand (0.17 EUR budget figures 2021 per share) The Group continues to New Council has been Group companies have successfully provide elected received Latvian supplies of goods in all Government financial operating markets support amounting to EUR 183 thousand Overall pharmaceutical Changes in Management Covid-19 restrictions markets in CIS region Board and election of new influenced possibilities of the continue to struggle Chairman of the Management Group to realize planned Board investments www.olainfarm.com 6 Main business factors Olainfarm products have shown strong demand due to product mix, brand recognition and renewed marketing strategy Business model restructuring in key markets are yielding remarkably good performance in Ukraine, Russia and Kazakhstan Devaluation of currencies in key markets was much milder than a year ago www.olainfarm.com 7 Financial performance in H1 2021 www.olainfarm.com 8 Key Financial Indicators Sales in H1 2021 showed sustainable growth due to strong brand -

Title of Project
USEAct Urban Sustainable Environmental Actions Fifth Meeting I Implementation Phase Riga Planning Region | Latvia 25th I 26th September 2014 INTERVENTIONS ON “REUSE” OF URBAN REFITTING AND REGENERATING INHABITED BUILDINGS AND AREAS Lead Partner Host Partner USEAct Riga Planning Region Fifth meeting Report Urban Sustainable Environmental Actions 2 Lead Partner City of Naples Urban Planning Department URBACT Projects_and Networks on Integrated Urban Development Policies - Central Direction Urban Planning and Management - UNESCO Site Gaetano Mollura USEAct Project coordinator Anna Arena Finance officer Maria Luna Nobile Communication officer Vincenzo Fusco LSG coordinator Contacts: phone +39 081 7958932 - 34 - 17 email [email protected] [email protected] Lead Expert Vittorio Alberto Torbianelli USEAct Project Lead Expert Contacts: email [email protected] Thematic Expert Pauline Geoghegan USEAct Project Thematic Expert Contacts: email [email protected] www.urbact.eu www.urbact.eu/useact The report written by the thematic expert Pauline Geoghegan NB. this report refers to the seminar work, with Should be read in contributions of Gaetano Mollura conjunction with the Lead partner, Vittorio Torbianelli Power Points Lead expert and USEAct partners presented during the meeting, that attended the meeting. Anna which you can Arena, Maria Luna Nobile and download here Vincenzo Fusco, Lead partner team contributed to the editing of this report. Cover picture: View of Riga city © Riga Planning Region All the photos are taken by the USEAct Team. And images are taken from the ppt presented during the seminar. Contents 1. Introduction and Concept paper 4 3 1.1 Introduction 1.2 Concept paper 1.3 The USEAct Issues of the Fifth Thematic Seminar 2.