Thermal Pasteurization of Ready-To-Eat Foods and Vegetables: Critical Factors for Process Design and Effects on Quality
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dairy and Food Sanitation 1982-10: Vol 2 Iss 10
Dairy and Food Sanitation Advertising Works... of Food and this is protection the p/ace to be for resuits ' Dairy Food sanitation Mail your copy or camera ready art work today to: lAMFES-Advertising P.O. Box 701 AMES, lA 50010 Ad placed in one publication Ad placed in both publications|(same month & copy) ADVERTISING RATES - Base Charge ADVERTISING RATES- Base Charge Black 8 white rates 1 time 6 times 12 times Black 8- white rates 1 time 6 times 12 times Back cover $715 $653 $591 Back Cover $476 $435 $393 Inside Back Cover 697 636 576 Inside Front Cover 464 425 383 Inside Front Cover 697 636 576 Inside Back Cover 464 425 383 One page 622 568 514 One page 414 378 342 2/3 page 472 422 378 2/3 page 315 281 252 1/2 page 416 332 1/2 page 252 222 195 380 1/3 page 270 244 216 1/3 page 180 162 145 1 /4 page 206 190 162 1/4 page 138 127 109 1 /8 page 151 126 109 1/8 page 100 84 73 2 color: Add $85 per placement Classified ads: 20C per word 4 color: Add $275 per placement Agency commission: 15% Bleed: Add $55 to your base charge Invoices due upon receipt MECHANICAL REQUIREMENTS CIRCULATION INFORMATION Full page 7" x 10" 2/3 page (horiz.) 7" x 614" 1/2 page (vert.) 3%" x 10" 1/2 page (horiz.) 7" x 5" 1/3 page (horiz.) 7" x 314" Major Responsibilities 1/4 page (vert.) 314"x4%" Milk and Food Quality Control . -

Pressure Canner and Cooker
Pressure Canner and Cooker Estas instrucciones también están disponibles en español. Para obtener una copia impresa: • Descargue en formato PDF en www.GoPresto.com/espanol. • Envíe un correo electrónico a [email protected]. • Llame al 1-800-877-0441, oprima 2 y deje un mensaje. For more canning information and recipes, visit www.GoPresto.com/recipes/canning Instructions and Recipes ©2019 National Presto Industries, Inc. Form 72-719J TABLE OF CONTENTS Important Safeguards.............................Below How to Can Foods Using Boiling Water Method .......... 21 Getting Acquainted .................................. 2 How to Pressure Cook Foods in Your Pressure Canner ....... 24 Before Using the Canner for the First Time................ 3 Important Safety Information ......................... 24 Canning Basics...................................... 4 Helpful Hints for Pressure Cooking..................... 25 How to Pressure Can Foods............................ 5 Pressure Cooking Meat .............................. 26 Troubleshooting ..................................... 7 Pressure Cooking Poultry ............................ 29 Care and Maintenance ................................ 7 Pressure Cooking Dry Beans and Peas .................. 30 Canning Fruits ...................................... 9 Pressure Cooking Soups and Stocks .................... 31 Canning Tomatoes and Tomato Products................. 12 Pressure Cooking Desserts............................ 32 Pressure Canning Vegetables .......................... 15 Recipe Index ..................................... -

REDUCTION of PURINE CONTENT in COMMONLY CONSUMED MEAT PRODUCTS THROUGH RINSING and COOKING by Anna Ellington (Under the Directio
REDUCTION OF PURINE CONTENT IN COMMONLY CONSUMED MEAT PRODUCTS THROUGH RINSING AND COOKING by Anna Ellington (Under the direction of Yen-Con Hung) Abstract The commonly consumed meat products ground beef, ground turkey, and bacon were analyzed for purine content before and after a rinsing treatment. The rinsing treatment involved rinsing the meat samples using a wrist shaker in 5:1 ratio water: sample for 2 or 5 minutes then draining or centrifuging to remove water. The total purine content of 25% fat ground beef significantly decreased (p<0.05) from 8.58 mg/g protein to a range of 5.17-7.26 mg/g protein after rinsing treatments. After rinsing and cooking an even greater decrease was seen ranging from 4.59-6.32 mg/g protein. The total purine content of 7% fat ground beef significantly decreased from 7.80 mg/g protein to a range of 5.07-5.59 mg/g protein after rinsing treatments. A greater reduction was seen after rinsing and cooking in the range of 4.38-5.52 mg/g protein. Ground turkey samples showed no significant changes after rinsing, but significant decreases were seen after rinsing and cooking. Bacon samples showed significant decreases from 6.06 mg/g protein to 4.72 and 4.49 after 2 and 5 minute rinsing and to 4.53 and 4.68 mg/g protein after 2 and 5 minute rinsing and cooking. Overall, this study showed that rinsing foods in water effectively reduces total purine content and subsequent cooking after rinsing results in an even greater reduction of total purine content. -

For Several Commercial Pasteurization and Sterilization Processes: Overview, Uses, and Restrictions
R&D AND PRODUCTION RETORTS SPECIALISTS (33 to 175 L) www.terrafoodtech.com Heat Process Values F (2nd Ed.) for several Commercial Pasteurization and Sterilization Processes: Overview, Uses, and Restrictions Janwillem Rouweler - [email protected]; June 12, 2015 Which heat process value F should a particular food receive to make it safe and shelf stable? 10 * Section 1 lists reported sterilization values F0 = F 121.1 (= F zero) for commercial food preservation processes of all types of food products, for several package sizes and types. * Section2 contains reported pasteurization values F, or P, of a great variety of foods. The required storage conditions of the pasteurized foods, either at ambient temperature, or refrigerated (4-7 °C), are indicated. * Section 3 shows a decision scheme: should a particular food be pasteurized or sterilized? This depends on the intended storage temperature (refrigerated or ambient) after heating, the required shelf life (7 days to 4 years), the food pH (high acid, acid, or low acid), the food - water activity aW, and on the presence of preservatives such as nitrite NO2 (E250) miXed with salt NaCl, or nisin. * In Section 4, two worked eXamples are presented on how to use an F value when calculating the actual sterilization time Pt: - C.R. Stumbo’s (1973) calculation method has been manually applied, verified by computer program STUMBO.eXe, to find the sterilization time and the thiamine retention of bottled liquid milk in a rotating steam retort; - O.T. Pham’s (1987; 1990) formula method, incorporated in EXcel program “Heat Process calculations according to Pham.xls”, has been used to find the sterilization time and the nutrient retention of canned carrot purée in a still steam retort. -

Braised Beef Short Ribs with Pomegranate Reduction from Serves (4) with (2) Short Ribs Per Person
Braised Beef Short Ribs with Pomegranate Reduction from www.farministasfeast.com serves (4) with (2) short ribs per person Ingredients: 2 tbsp olive oil 3 pounds beef short ribs (bone in ok) 1/2 cup all purpose flour* fresh ground sea salt & pepper 1 1/2 cups pomegranate juice 1/4 cup pomegranate vinegar 4 tbsp dark brown sugar mashed potatoes or cauliflower mash *you may skip the flour (or substitute rice flower) and brown the short ribs seasoned with salt and pepper for a gluten-free preparation. Directions: Preheat the oven to 300F. Combine the flour, salt and pepper in a large gallon-size ziplock bag. Add the short ribs, seal, and shake the bag to coat the meat with flour mixture. Heat the olive oil a large heavy skillet (preferably cast iron) on the stove over medium heat. Shake off any excess flour from each short rib, and place each rib in the hot oil. Sear on all sides until light golden brown (about a minute or two per side). Remove browned ribs from the pan and set aside. In a small sauce pan, combine the pomegranate juice, pomegranate vinegar and brown sugar over medium heat. Simmer and stir juice until the brown sugar is melted. Remove pan from the heat and aside. Place the browned short ribs in a dutch oven or ceramic casserole (with a lid). Pour the pomegranate juice mixture over the top of the ribs. Cover the dish with a lid and place on the center oven rack for 2 1/2 to 3 hours to braise. -

Technique of the Quarter: Examining Sauces
TECHNIQUE OF THE QUARTER: EXAMINING SAUCES Sauces are often considered one of the greatest tests of a chef’s skill. The successful pairing of a sauce with a food demonstrates technical expertise, an understanding of the food, and the ability to judge and evaluate a dish’s flavors, textures, and colors. THE PURPOSE OF SAUCES Most sauces have more than one function in a dish. A sauce that adds a counterpoint flavor, for example, may also introduce textural and visual appeal. Sauces generally serve one or more of the following purposes. Introduce Complementary or Contrasting Flavors Sauces add flavor to a dish. That flavor can be similar to the flavor of the food you are serving it with. For instance, you might choose a velouté made with chicken stock to serve with a chicken breast dish and one made with shellfish stock to serve with a shrimp dish. Choosing a sauce with a similar base flavor tends to complement and intensify the flavor of the main item. On the other hand, you can choose a sauce that adds a contrasting flavor. A good example would be a red wine sauce that introduces some bright and acidic flavors to a dish that features beef. The contrast between rich, savory beef flavors and the sharp taste of the wine makes the beef stand out. Add Moisture A sauce can add moisture to naturally lean foods such as poultry, fish. A sauce can also compensate for the drying effect of certain cooking techniques, especially broiling, grilling, sautéing, and roasting. Grilled foods may be served with a warm butter emulsion sauce like béarnaise or with compound butter. -
Cooking Methods
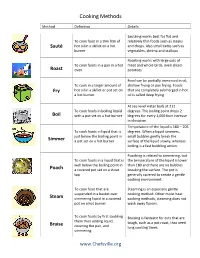
Cooking Methods Method Definition Details Sautéing works best for flat and To cook food in a thin film of relatively thin foods such as steaks Sauté hot oil in a skillet on a hot and chops. Also small items such as burner vegetables, shrimp and scallops Roasting works with large cuts of To cook foods in a pan in a hot meat and whole birds, even sliced Roast oven potatoes Food can be partially immersed in oil, To cook in a larger amount of shallow frying or pan frying. Foods Fry hot oil in a skillet or pot set on that are completely submerged in hot a hot burner oil is called deep frying. At sea level water boils at 212 To cook foods in boiling liquid degrees. The boiling point drops 2 Boil with a pot set on a hot burner degrees for every 1,000 foot increase in elevation. Temperature of the liquid is 180 – 205 To cook foods in liquid that is degrees. When a liquid simmers, just below the boiling point in small bubbles gently break the Simmer a pot set on a hot burner surface of the liquid slowly, whereas boiling is a fast bubbling action. Poaching is related to simmering, but To cook foods in a liquid that is the temperature of the liquid is lower well below the boiling point in than 180 and there are no bubbles Poach a covered pot set on a stove breaking the surface. The pot is top generally covered to create a gentle cooking environment. To cook food that are Steaming is an especially gentle suspended in a basket over cooking method. -

Simple Suppers: Findings from a Family Meals Childhood Obesity Prevention Intervention
Simple Suppers: Findings from a Family Meals Childhood Obesity Prevention Intervention Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Catherine Ann Rogers, MS, RDN, LD Ohio State University Nutrition Graduate Program The Ohio State University 2017 Dissertation Committee: Carolyn W. Gunther, PhD (Advisor) Sarah Anderson, PhD Carla Miller, PhD, RD Keeley Pratt, PhD, IMFT Copyright by Catherine Ann Rogers 2017 Abstract Background: Given the ongoing childhood obesity public health crisis and potential protective effect of family meals, there is need for additional family meals research specifically experimental studies with expanded health outcomes that focus on the at-risk populations in highest need of intervention. Future research, specifically intervention work, would also benefit from an expansion of the target age rage to include younger children who are laying the foundation of their eating patterns and are capable of participating in family meal preparations. The purpose of this dissertation research was to address this research gap by developing and assessing the effectiveness of a 10-week multi-component family meals intervention targeting underserved families with children 4-10 years old, aimed at eliciting positive changes in child diet and weight status. Methods: A 10-week family meals program (Simple Suppers) designed for underserved families with 4-10 year old children from racially diverse backgrounds was implemented as a pre-test-post-test, multi-cohort, quasi-experimental trial with waitlist control. The 10, 90-minute program lessons were delivered weekly over the dinner hour at a faith- based community center. -

COLORING MATTER of RAW and COOKED SALTED MEATS the Red
COLORING MATTER OF RAW AND COOKED SALTED MEATS By RALPH HOAGLAND, Laboratory Inspector, Biochemie Division, Bureau of Animal Industry INTRODUCTION The red color of fresh lean meat, such as beef, pork, and mutton, is due to the presence of oxyhemoglobin, a part of which is one of the con- stituents of the blood remaining in the tissues, while the remainder is a normal constituent of the muscles. When fresh meat is cooked or is cured by sodium chlorid, the red color changes to brown, owing to the breaking down of the oxyhemoglobin into the two constituents, hematin, the coloring group, and the proteid, globin. On the other hand, when fresh meat is cured by means of a mixture of sodium chlorid and a small proportion of potassium nitrate, or salt- peter, either as a dry mixture or in the form of a pickle, the red color of the fresh meat is not destroyed during the curing process, the finished product having practically the same color as the fresh meat. Neither is the red color destroyed on cooking, but rather is intensified. The practical value of saltpeter in the curing of meats is so well known that its use for this purpose may be said to have become practically uni- versal ; such use is not confined to the commercial meat-packing industry, but it is used in the home curing of meats as well. It is only within comparatively recent years, however, that anything very definite has been known concerning the nature of the color of salted meats or the process of the color formation. -

Meals for Weight Reduction
Meals for Weight Reduction GA Foods’ SunMeadow® meals are designed to provide optimal nutrition for good health. These meals are all <650 calories, >20 g protein, <35% calories from fat, minimized refined sugar, and >7 g fiber. TMS001361 Chargrilled Beef Patty in Pizzaiola Sauce, Pancakes and Egg Patty, served with turkey served with green beans, pineapple Mandarin sausage links, strawberry compote, and whole oranges, and whole wheat bread. grain bread. Chicken Garden Casserole, served with Meatloaf with Apple Brown Gravy, served with summer blend vegetables, pineapple cup, skin-on potatoes, stewed tomatoes, pineapple and whole wheat bread. cup, and whole wheat bread. Macaroni and Beef Casserole, served with Oven Baked Chicken, served with Southern spinach, California blend vegetables, pear cup, rice & black-eyed peas, spinach, Mandarin and whole wheat bread. oranges, and whole wheat bread. Sliced Turkey and Gravy, served with cornbread dressing, flat beans, butternut squash, citrus fruit cup, and whole wheat bread. TMS001362 Sweet and Sour Chicken, steamed white rice, gingeredserved peas, with Chicken Marsala, served with green beans, Mandarin oranges, and whole wheat bread. butternut squash, mixed fruit cup, and whole wheat bread. Hamburger Patty Au Jus, served with mashed potatoes, carrots, applesauce, and whole Grilled Veal Chop with Mustard Sage Sauce, grain bun. served with diced potatoes, maple butternut squash, pear cup, and whole wheat bread. Lasagna (with ground turkey), served with broccoli, cauliflower & bean medley, peach Western-Style Omelet, cup, and whole wheat bread. O’Brien, strawberry applesauce,served with whole potatoes wheat bread, and graham crackers. Sliced Roast Beef with Gravy, spring peas, buttered crinkle cut served carrots, with raisins, and whole wheat bread. -

A Centurty of Food Science IFT Booklet
This book was the product of the Research Report Task Force, including Roy G. Arnold Robert E. Berry Ellen Bradley Walter L. Clark AI. S. Clausi Arnold E. Denton Charles Feldberg F. Jack Francis Daniel Fung Marianne Gillette Dennis Heldman Richard Lechowich Gilbert Leveille David Lineback Daryl Lund Laszlo P. Somogyi Special thanks to three 1FT members who wrote special articles about their acquaintences and experiences: Al Clausi John Powers Jack Francis Editing by 1FT Staff copywrite 2000 ood science is a somewhat odd amalgam of scientific disciplines, including basic sciences, "soft" science, culinary arts and its out• growths, chemistry, biology, economics, agronomics, microbiology, and engineering. There are others, but "food science" is the common theme that turns traditional foods into a variety of specialty products and makes them tasty, safe, available, and convenient. Food supply and security are sometimes prominent in the roster, and other disciplines take their place from time to time. It is rare that food science teaches something truly new-but the "devil is in the details," and food science takes knowledge to a supremely practical use. Between the early 1900s and the present, food science and its cousins have provided Americans with the safest food supply the world has ever known, as well as the most plentiful and the least expensive. The other developed countries of the world have also been provided with the same kind of food supply. The underdeveloped and developing countries of the world have an improving food situation, and the information gained during the long race toward food security for developing countries has been extremely helpful to developed countries as well. -

Principles of the Food Processing & Preservation
Paper No.: 02 Paper Title: PRINCIPLES OF THE FOOD PROCESSING & PRESERVATION Module – 06: Thermal Death Time Curves Paper Coordinator: Dr. P. Narender Raju, Scientist, National Dairy Research Institute, Karnal, Haryana Content Writer: Dr. Kuna Aparna, Asst. Professor, Prof. Jayashankar Telangana State Agricultural University, Hyderabad Module 6 THERMAL DEATH TIME CURVES Introduction • Thermal calculations thus involve the need for knowledge of the concentration of microorganisms to be destroyed, the acceptable concentration of microorganisms that can remain behind (spoilage organisms, for example, but not pathogens), the thermal resistance of the target microorganisms (the most heat tolerant ones), and the temperature time relationship required for destruction of the target organisms. • The extent of the pasteurization treatment required is determined by the heat resistance of the most heat-resistant enzyme or microorganism in the food. • A thermal death curve for this process is shown in next slide. It is a logarithmic process, meaning that in a given time interval and at a given temperature, the same percentage of the bacterial population will be destroyed regardless of the population present. • Several parameters help us to do thermal calculations and define the rate of thermal lethality. The D value is a measure of the heat resistance of a microorganism. It is the time in minutes at a given temperature required to destroy 1 log cycle (90%) of the target microorganism. (Of course, in an actual process, all others that are less heat tolerant are destroyed to a greater extent). For example, a D value at 72°C of 1 minute means that for each minute of processing at 72°C the bacteria population of the target microorganism will be reduced by 90%.