COLORING MATTER of RAW and COOKED SALTED MEATS the Red
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pressure Canner and Cooker
Pressure Canner and Cooker Estas instrucciones también están disponibles en español. Para obtener una copia impresa: • Descargue en formato PDF en www.GoPresto.com/espanol. • Envíe un correo electrónico a [email protected]. • Llame al 1-800-877-0441, oprima 2 y deje un mensaje. For more canning information and recipes, visit www.GoPresto.com/recipes/canning Instructions and Recipes ©2019 National Presto Industries, Inc. Form 72-719J TABLE OF CONTENTS Important Safeguards.............................Below How to Can Foods Using Boiling Water Method .......... 21 Getting Acquainted .................................. 2 How to Pressure Cook Foods in Your Pressure Canner ....... 24 Before Using the Canner for the First Time................ 3 Important Safety Information ......................... 24 Canning Basics...................................... 4 Helpful Hints for Pressure Cooking..................... 25 How to Pressure Can Foods............................ 5 Pressure Cooking Meat .............................. 26 Troubleshooting ..................................... 7 Pressure Cooking Poultry ............................ 29 Care and Maintenance ................................ 7 Pressure Cooking Dry Beans and Peas .................. 30 Canning Fruits ...................................... 9 Pressure Cooking Soups and Stocks .................... 31 Canning Tomatoes and Tomato Products................. 12 Pressure Cooking Desserts............................ 32 Pressure Canning Vegetables .......................... 15 Recipe Index ..................................... -

REDUCTION of PURINE CONTENT in COMMONLY CONSUMED MEAT PRODUCTS THROUGH RINSING and COOKING by Anna Ellington (Under the Directio
REDUCTION OF PURINE CONTENT IN COMMONLY CONSUMED MEAT PRODUCTS THROUGH RINSING AND COOKING by Anna Ellington (Under the direction of Yen-Con Hung) Abstract The commonly consumed meat products ground beef, ground turkey, and bacon were analyzed for purine content before and after a rinsing treatment. The rinsing treatment involved rinsing the meat samples using a wrist shaker in 5:1 ratio water: sample for 2 or 5 minutes then draining or centrifuging to remove water. The total purine content of 25% fat ground beef significantly decreased (p<0.05) from 8.58 mg/g protein to a range of 5.17-7.26 mg/g protein after rinsing treatments. After rinsing and cooking an even greater decrease was seen ranging from 4.59-6.32 mg/g protein. The total purine content of 7% fat ground beef significantly decreased from 7.80 mg/g protein to a range of 5.07-5.59 mg/g protein after rinsing treatments. A greater reduction was seen after rinsing and cooking in the range of 4.38-5.52 mg/g protein. Ground turkey samples showed no significant changes after rinsing, but significant decreases were seen after rinsing and cooking. Bacon samples showed significant decreases from 6.06 mg/g protein to 4.72 and 4.49 after 2 and 5 minute rinsing and to 4.53 and 4.68 mg/g protein after 2 and 5 minute rinsing and cooking. Overall, this study showed that rinsing foods in water effectively reduces total purine content and subsequent cooking after rinsing results in an even greater reduction of total purine content. -

PPI for Meats, Poultry, and Fish
PPI for Meats, poultry, and fish Nov 13, 2019 2 https://www.GetArgon.io 3 PPI (historical) for meats, poultry, and fish PPI or Producer Price Index (also known as wholesale price index) for meats, poultry, and fish. PPI data is normalized with price for 1982 = 100. The PPI value presented here is the average of the monthly value for each year. Includes the following: meats, beef and veal products, fresh or frozen, beef, fresh/frozen variety meats, not canned or made into sausage, made in slaughtering plants, veal, fresh or frozen, not canned or sausage, made in slaughtering plants, beef, fresh/frozen whole/half carcass, not canned or made into sausage, misp, beef, fresh/ frozen, primal and subprimal cuts, made in slaughtering plants, lamb/mutton, fresh or frozen, not canned or made into sausage, made in slaughtering plants, pork products, fresh, frozen, or processed, except sausage, pork, fresh/ frozen, unprocessed, all cuts, except sausage, made in slaughtering plants, pork, processed or cured, not canned or made into sausage, pork, processed or cured, not canned or made into sausage, made in slaughtering plants, pork, processed or cured, not canned or made into sausage, mfpc, pork, sweet-pickled, dry-cured, or dry salt, not canned or made into sausage, mfpc, smoked hams and picnics, excluding canned, made from purchased carcasses, smoked slab bacon, made from purchased carcasses, smoked sliced bacon, made from purchased carcasses, other smoked pork, not canned or made into sausage, made from purchased carcasses, boiled ham, barbecue pork, -

For Several Commercial Pasteurization and Sterilization Processes: Overview, Uses, and Restrictions
R&D AND PRODUCTION RETORTS SPECIALISTS (33 to 175 L) www.terrafoodtech.com Heat Process Values F (2nd Ed.) for several Commercial Pasteurization and Sterilization Processes: Overview, Uses, and Restrictions Janwillem Rouweler - [email protected]; June 12, 2015 Which heat process value F should a particular food receive to make it safe and shelf stable? 10 * Section 1 lists reported sterilization values F0 = F 121.1 (= F zero) for commercial food preservation processes of all types of food products, for several package sizes and types. * Section2 contains reported pasteurization values F, or P, of a great variety of foods. The required storage conditions of the pasteurized foods, either at ambient temperature, or refrigerated (4-7 °C), are indicated. * Section 3 shows a decision scheme: should a particular food be pasteurized or sterilized? This depends on the intended storage temperature (refrigerated or ambient) after heating, the required shelf life (7 days to 4 years), the food pH (high acid, acid, or low acid), the food - water activity aW, and on the presence of preservatives such as nitrite NO2 (E250) miXed with salt NaCl, or nisin. * In Section 4, two worked eXamples are presented on how to use an F value when calculating the actual sterilization time Pt: - C.R. Stumbo’s (1973) calculation method has been manually applied, verified by computer program STUMBO.eXe, to find the sterilization time and the thiamine retention of bottled liquid milk in a rotating steam retort; - O.T. Pham’s (1987; 1990) formula method, incorporated in EXcel program “Heat Process calculations according to Pham.xls”, has been used to find the sterilization time and the nutrient retention of canned carrot purée in a still steam retort. -

Braised Beef Short Ribs with Pomegranate Reduction from Serves (4) with (2) Short Ribs Per Person
Braised Beef Short Ribs with Pomegranate Reduction from www.farministasfeast.com serves (4) with (2) short ribs per person Ingredients: 2 tbsp olive oil 3 pounds beef short ribs (bone in ok) 1/2 cup all purpose flour* fresh ground sea salt & pepper 1 1/2 cups pomegranate juice 1/4 cup pomegranate vinegar 4 tbsp dark brown sugar mashed potatoes or cauliflower mash *you may skip the flour (or substitute rice flower) and brown the short ribs seasoned with salt and pepper for a gluten-free preparation. Directions: Preheat the oven to 300F. Combine the flour, salt and pepper in a large gallon-size ziplock bag. Add the short ribs, seal, and shake the bag to coat the meat with flour mixture. Heat the olive oil a large heavy skillet (preferably cast iron) on the stove over medium heat. Shake off any excess flour from each short rib, and place each rib in the hot oil. Sear on all sides until light golden brown (about a minute or two per side). Remove browned ribs from the pan and set aside. In a small sauce pan, combine the pomegranate juice, pomegranate vinegar and brown sugar over medium heat. Simmer and stir juice until the brown sugar is melted. Remove pan from the heat and aside. Place the browned short ribs in a dutch oven or ceramic casserole (with a lid). Pour the pomegranate juice mixture over the top of the ribs. Cover the dish with a lid and place on the center oven rack for 2 1/2 to 3 hours to braise. -

1,500 Types of Sausage 1 Scalded - Put Into Boiling Water and Heat~ to Taste - a Range Which Includes Frankfurter and Wiener
1,500 Types of Sausage 1 Scalded - put into boiling water and heat~ to taste - a range which includes Frankfurter and Wiener. The latter takes its name from a Wurst citizen of Berlin, who had nothing to do with Austria's capital. The sausage his inventive mind Did you know that strict German laws affecting thought up is rather like a Frankfurter. trade descriptions allow only Frankfurters fr0m WeiBwurst, another sea lded sausage, comes Frankfurt to be called Frankfurter? That in fr0m Munich, and is fat and greyish-white !n Germany a Hamburger is a citizen of Hamburg, ool0ur. Bockwurst is found almost everywhere, not a piece of minced beef - which in Germany and is recognisable by its short, fat sha@e. is called a Deutsches Beefsteak? That the Ger Bratwurst, originally a product of Ni.irnberg, is mans eat much more pork than any other now popular throughout Germany. When grilled meat? Orthat some Germans find the idea over an open fire it is called Rostbratwurst. 0f eating lamb somewhat distasteful? 2 Boiled (eaten cold): the two best-known Buying meat is no problem in Germany; in fact boiled varieties are Leberwurst (liver sausage), a meat can even be bought at an inn. Many older soft sausage easy to spread on bread, and inns, especially •in the soutli, have their own Blutwurst (something like black pudding) butcher's shop in one part of the building, which is often heavily spiced. which is then called a Gasthaus-Metzgerei. 3 Smoked: smoked sausages are mostly lean It is Wurst - the huge range of German cold and rather dry, with good keeping qu.i lities. -

Catering Menu
Appetizer Trays Dessert Trays International Sausage & Cheese All trays 5 person minimum Platter Fresh-Baked Cookies - $1.75 A selection of European-style sausages Assortment of chocolate chunk, oatmeal JJ that includes Polish kielbasa, Ukrainian raisin and sugar cookies. kielbasa, German hunter sausage & veal Fresh-Baked Cookies & Brownies International & pork kabanos. Cheese selection $2.25 Delicatessen includes mild Dutch Edam, Ukrainian Assortment of chocolate chunk, oatmeal Abbatsky, NY State cheddar, Polish raisin, sugar cookies, chewy Rocky Road & Catering Holldamer, Ukrainian smoked Swiss. chocolate chip brownies. $4.75 per person, 5 person Polish-Style Cheese Cake - $2.50 Menu minimum A dense ricotta cheese cake with peaches Polish Marble Cake - $2.5 International Sausage, Salami & Delicious, light marble cake Cheese Platter Assorted Cakes Tray - $2.50 A selection of European-style sausages & Our marble cake, ricotta cheese cake & salamis that includes Polish kielbasa, chewy brownies 174 Pike Street Ukrainian kielbasa, German hunter (in between Dad’s & the sausage & veal & pork kabanos, What Makes us Laundromat) German Cerevlat salami, Paris salami, Port Jervis, NY Genoa salami. Cheese selection includes Unique? (845)858-1142 mild Dutch Edam, Ukrainian, Abbatsky, We start with the finest, freshest NY State cheddar, Polish Holldamer, ingredients such as our in-house roasted, We Accept All Major Credit Ukrainian smoked Swiss. seasoned top round, authentic Polish Cards $5.00 per person, 5 person kielbasa, delicious Polish ham, imported Delivery Can be Arranged, minimum cheeses and the best homemade stuffed Port Jervis, Matamoras and International Cheese Platter cabbage in Tri-States. All of our meats are Immediate Surrounding Area Choose from our 28 cheeses to make it from smaller processors and in some cases still made by hand, such as our delicious Tuesday – Friday, 9AM – 6PM your own! Saturday 9AM – 5PM $4.25 per person, 5 person minimum Forest Pork Store offerings. -

Chapter 18 : Sausage the Casing
CHAPTER 18 : SAUSAGE Sausage is any meat that has been comminuted and seasoned. Comminuted means diced, ground, chopped, emulsified or otherwise reduced to minute particles by mechanical means. A simple definition of sausage would be ‘the coarse or finely comminuted meat product prepared from one or more kind of meat or meat by-products, containing various amounts of water, usually seasoned and frequently cured .’ In simplest terms, sausage is ground meat that has been salted for preservation and seasoned to taste. Sausage is one of the oldest forms of charcuterie, and is made almost all over the world in some form or the other. Many sausage recipes and concepts have brought fame to cities and their people. Frankfurters from Frankfurt in Germany, Weiner from Vienna in Austria and Bologna from the town of Bologna in Italy. are all very famous. There are over 1200 varieties world wide Sausage consists of two parts: - the casing - the filling THE CASING Casings are of vital importance in sausage making. Their primary function is that of a holder for the meat mixture. They also have a major effect on the mouth feel (if edible) and appearance. The variety of casings available is broad. These include: natural, collagen, fibrous cellulose and protein lined fibrous cellulose. Some casings are edible and are meant to be eaten with the sausage. Other casings are non edible and are peeled away before eating. 1 NATURAL CASINGS: These are made from the intestines of animals such as hogs, pigs, wild boar, cattle and sheep. The intestine is a very long organ and is ideal for a casing of the sausage. -

Technique of the Quarter: Examining Sauces
TECHNIQUE OF THE QUARTER: EXAMINING SAUCES Sauces are often considered one of the greatest tests of a chef’s skill. The successful pairing of a sauce with a food demonstrates technical expertise, an understanding of the food, and the ability to judge and evaluate a dish’s flavors, textures, and colors. THE PURPOSE OF SAUCES Most sauces have more than one function in a dish. A sauce that adds a counterpoint flavor, for example, may also introduce textural and visual appeal. Sauces generally serve one or more of the following purposes. Introduce Complementary or Contrasting Flavors Sauces add flavor to a dish. That flavor can be similar to the flavor of the food you are serving it with. For instance, you might choose a velouté made with chicken stock to serve with a chicken breast dish and one made with shellfish stock to serve with a shrimp dish. Choosing a sauce with a similar base flavor tends to complement and intensify the flavor of the main item. On the other hand, you can choose a sauce that adds a contrasting flavor. A good example would be a red wine sauce that introduces some bright and acidic flavors to a dish that features beef. The contrast between rich, savory beef flavors and the sharp taste of the wine makes the beef stand out. Add Moisture A sauce can add moisture to naturally lean foods such as poultry, fish. A sauce can also compensate for the drying effect of certain cooking techniques, especially broiling, grilling, sautéing, and roasting. Grilled foods may be served with a warm butter emulsion sauce like béarnaise or with compound butter. -
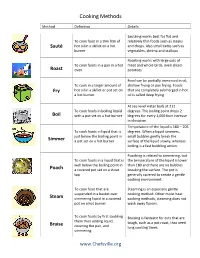
Cooking Methods
Cooking Methods Method Definition Details Sautéing works best for flat and To cook food in a thin film of relatively thin foods such as steaks Sauté hot oil in a skillet on a hot and chops. Also small items such as burner vegetables, shrimp and scallops Roasting works with large cuts of To cook foods in a pan in a hot meat and whole birds, even sliced Roast oven potatoes Food can be partially immersed in oil, To cook in a larger amount of shallow frying or pan frying. Foods Fry hot oil in a skillet or pot set on that are completely submerged in hot a hot burner oil is called deep frying. At sea level water boils at 212 To cook foods in boiling liquid degrees. The boiling point drops 2 Boil with a pot set on a hot burner degrees for every 1,000 foot increase in elevation. Temperature of the liquid is 180 – 205 To cook foods in liquid that is degrees. When a liquid simmers, just below the boiling point in small bubbles gently break the Simmer a pot set on a hot burner surface of the liquid slowly, whereas boiling is a fast bubbling action. Poaching is related to simmering, but To cook foods in a liquid that is the temperature of the liquid is lower well below the boiling point in than 180 and there are no bubbles Poach a covered pot set on a stove breaking the surface. The pot is top generally covered to create a gentle cooking environment. To cook food that are Steaming is an especially gentle suspended in a basket over cooking method. -

Simple Suppers: Findings from a Family Meals Childhood Obesity Prevention Intervention
Simple Suppers: Findings from a Family Meals Childhood Obesity Prevention Intervention Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Catherine Ann Rogers, MS, RDN, LD Ohio State University Nutrition Graduate Program The Ohio State University 2017 Dissertation Committee: Carolyn W. Gunther, PhD (Advisor) Sarah Anderson, PhD Carla Miller, PhD, RD Keeley Pratt, PhD, IMFT Copyright by Catherine Ann Rogers 2017 Abstract Background: Given the ongoing childhood obesity public health crisis and potential protective effect of family meals, there is need for additional family meals research specifically experimental studies with expanded health outcomes that focus on the at-risk populations in highest need of intervention. Future research, specifically intervention work, would also benefit from an expansion of the target age rage to include younger children who are laying the foundation of their eating patterns and are capable of participating in family meal preparations. The purpose of this dissertation research was to address this research gap by developing and assessing the effectiveness of a 10-week multi-component family meals intervention targeting underserved families with children 4-10 years old, aimed at eliciting positive changes in child diet and weight status. Methods: A 10-week family meals program (Simple Suppers) designed for underserved families with 4-10 year old children from racially diverse backgrounds was implemented as a pre-test-post-test, multi-cohort, quasi-experimental trial with waitlist control. The 10, 90-minute program lessons were delivered weekly over the dinner hour at a faith- based community center. -

Meat Preservation
Meat Preservation Sausage Objectives •Exppgplain methods for sausage production • Examine the variety of meats made through sausage production What is a Sausage? • Word sausage is from word Salsus – Salsus means salt or preserved • Is a chopped or comminuted and seasoned meat • Can be: –Fresh –Cured – Smoked – Heat processed • Contain non-meat ingredients History of Sausage • Reason for discovery of America and trade with Asia • Used to be “Bags of Mystery” – Historically were made from by -products and left-overs • Modern sausage is made from lean trimmings – Cheek, jowl , and heads from beef pork and poultry Why are sows used for Sausage? Types of Sausage Sausage classification Characteristics Examples Fresh sausage Uncooked, uncured fresh meats, Bulk fresh pork chopped, seasoned and usually sausage, country-style put in casings pork sausage, bratwurst Dry and semidry Cured meats, dried, may be Pepperoni, salami, sausages smoked cervelat Cooked sausages Meat cured or uncured, chopped, Liver cheese, seasoned, cooked and sometimes braunschweiger, liver smoked sausage Cooked, smoked Cured meat, chopped, seasoned, Bologna, wiener, cotto sausages smoked and cooked salami Uncooked, smoked Fresh meat, cured or uncured, Smoked country-style sausages smoked and must be cooked pork sausage, smokies, before serving mettwurst Luncheon meats Often made into loaves but Chopped ham, blood and and loaves sliced, cured or uncured meat, tongue pudding, head cooked but usually not smoked cheese Sausage Varieties Common Sausage Varieties • Luncheon Meats (bologna) – Modern use of pork trimmings – Comminuted or chopped – Contains few seasonings Uncommon Sausage Varieties • Head Cheese – Mixture of meats held together by a gelatin – Used in salads, sandwiches and as hors d’oeuvres – Traditional meats Consumption of Sausage Sausage Ingredients • Animal Tissues- – A.