Assessment of Seasonal Variations in Physico-Chemical Parameters in Panchet Reservoir, Dhanbad District, Jharkhand
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Report 2 0 1 2 - 1 3
Annual Report 2 0 1 2 - 1 3 Ministry of Power Government of India Shram Shakti Bhawan, Rafi Marg, New Delhi-110 001 Website : www.powermin.nic.in Shri Pranab Mukherjee, Hon’ble President of India with Shri Jyotiraditya M. Scindia, Hon’ble Union Minister of State for Power (Independent Charge) at the National Energy Conservation Day function CONTENTS Sl. No. Chapter Page No. (s) 1. Performance Highlights 5 2. Organisational Set Up and Functions of the Ministry of Power 9 3. Capacity Addition Programme in the XIIth Plan 11 4. Generation & Power Supply Position 23 5. Status of Ultra Mega Power Projects 35 6. Transmission 37 7. Status of Power Sector Reforms 41 8. Rural Electrification Programme 43 9. Re-Structured Accelerated Power Development and Reforms Programme (R-APDRP) 45 10. Energy Conservation 49 11. Renovation and Modernisation of Thermal Power Stations 53 12. Private Sector Participation in Power Sector 57 13. International Cooperation 59 14. Power Development Activities in North-Eastern Region 67 15. Central Electricity Authority 75 16. Central Electricity Regulatory Commission (CERC) 79 17. Appellate Tribunal for Electricity (APTEL) 83 Public Sector Undertakings: 18 NTPC Limited 85 19. NHPC Limited 105 20. Power Grid Corporation of India Ltd. (PGCIL) 111 21. Power Finance Corporation Ltd. (PFC) 115 22. Rural Electrification Corporation Ltd. (REC) 125 23. North Eastern Electric Power Corporation Limited (NEEPCO) 133 Joint Venture Corporations : 24. SJVN Limited (SJVNL) 135 25. THDC India Limited (THDCIL) 139 Statutory Bodies : 26. Damodar Valley Corporation (DVC) 143 27. Bhakra Beas Management Board (BBMB) 149 28. Bureau of Energy Efficiency (BEE) 155 Autonomous Bodies : 29. -

Access Jharkhand-Obj07-04-2021-E-Book
Index 01. Jharkhand Special Branch Constable (Close 16. JSSC Assistant Competitive Examination Cadre) Competitive Exam 01-09-2019 28.06.2015. 02. J.S.S.C. - Jharkhand Excise Constable Exam 17. Jharkhand Forest Guard Appointment Com- 04-08-2019 petitive (Prelims) Exam - 24.05.2015. 03. SSC IS (CKHT)-2017, Intermediate Level (For 18. Jharkhand Staff Selection Commission the post of Hindi Typing Noncommittee in Com- organized Women Supervisor competitive puter Knowledge and Computer) Joint Competi- Exam - 2014. tive Exam 19. Fifth Combined Civil Service Prelims Compet- 04. JUVNL Office Assistent Exam 10-03-2017 itive Exam - 15.12.2013. 05. J.S.S.C. - Post Graduate Exam 19-02-2017 20. Jharkhand Joint Secretariat Assistant (Mains) 06. J.S.S.C Amin Civil Resional Investigator Exam Examination 16.12.2012. 08-01-2017 21. State High School Teacher Appointment 07. JPSC Prelims Paper II (18.12.2016) Examination 29.08.2012. 08. JPSC Prelims Paper-I (Jharkhand Related 22. Jharkhand Limited Departmental Exam- Questions Only on 18.12.2016) 2012. 09. Combined Graduation Standard Competitive 23. Jharkhand Joint Secretariat Assistant Exam- (Prelims) Examinations 21.08.2016 2012. 10. Kakshpal appointment (mains) Competitive 24. Fourth Combined Civil Service (Prelims) Examination 10.07.2016. Competitive Examination - 2010. 11. Jharkhand Forest guard appointment (mains) 25. Government High School Teacher Appoint- Competitive Examination 16.05.2016. ment Exam - 2009. 12. JSSC Kakshpal Competitive (Prelims) Exam - 26. Primary Teacher Appointment Exam - 2008. 20.03.2016. 27. Third Combined Civil Service Prelims 13. Jharkhand Police Competitive Examination Competitive Exam - 2008. 30.01.2016. 28. JPSC Subsidiary Examination - 2007. -

Damodar : a River Valley of Sorrow in Jharkhand State of India The
Damodar : A River Valley of Sorrow in Jharkhand state of India The Damodar is an inter-state river in the state of Jharkhand in India. In general rivers are feminine but Damodar is an exception. Like other two rivers -The Sonebhadra and The Brahmaputra Damodar is also categorised as a Masculine River. It emerges from the roots of an old tree, know as Pakar tree in local dialect, of extremists infested Boda Hills at "Kuru" Block of "Lohardaga " District in Jharkhand and merges into River "Bhagirathi " after traversing a total length of 541 Kms of which 258 Kms lies in the Jharkhand and the rest in West Bengal province. The total catchments area of the Damodar river system is 22,528 Sq Kms of which 16,934 Sq Kms (76 percent) is in the state of Jharkhand. The average yield of the Damodar River basin is 12.20 and its total surface flow in Jharkhand is estimated to be 5.80 Lham at 75 percent dependability as reported by the Irrigation Commission, Govt. of India 1972. Tributaries :- Its important tributaries are Barakar, Konar Bokaro and Gowai. The "barakar river " is its main tributary running almost parallel to it and joins it at 258 Kms near panchet at the border of Jharkhand and west Bengal where as its another left bank tributary "river Konar " merges into it at 180 kms from its origin near Bermo in Bokaro district of Jharkhand. The Bokaro and Konar rivers rise very near to each other on the Hazaribagh plateau and the two together meet meet before they finally outfall into Damodar at above 5 Kms further downstream. -

Action Plan for Clusters of Dhanbad
ACTION PLAN FOR CLUSTERS OF DHANBAD _________________________________________________ JHARKHAND STATE POLLUTION CONTROL BOARD T A BUILDING, HEC COMPLEX, RANCHI 834004 1 (1) 1.0 INTRODUCTION 1.1 The territorial area of Nirsa block , industrial area of Govindpur block, municipal area of Dhanbad Sadar block, municipal area of Jharia block and industrial area of Sindri are included in the cluster of Dhanbad 1.2 Location: Satellite imagery is as given below. SATELLITE IMAGERY OF INDUSTRIAL CLUSTER OF DHANBAD SATELLITE IMAGERY OF THE AREA OF NIRSA BLOCK 2 SATELLITE IMAGERY OF DHANBAD SADAR BLOCK SATELLITE IMAGERY OF INDUSTRIAL AREA OF SINDRI 3 SATELLITE IMAGERY OF JHARIA BLOCK SATELLITE IMAGERY OF THE AREA OF GOBINDPUR 1.3 Digital map with demarcation of geographical boundaries and impact zones is as given below. 4 1.4 CEPI Score: Air- 64.50, Water- 59.00, Land -65.50, Total- 78.63 1.5 Total populations and sensitive receptors Hospital – Patliputra Medical College & Hospital, Dhanbad;Central Hospital, Jagjivan Nagar; Jamadoba Hospital; Fertilizer Hospital, Sindri Educational Institution – BIT Sindri, ISM Dhanbad, Patliputra Medical College, Dhanbad, RS More College, Govindpur, Sindri College, RSP College, Jharia, PK Roy College, SSLNT Womens College, BS College, Govt. Polytechnic, Dhanbad, Mining Institute, Dhanbad, Govt. Polytechnic, Bhaga; Mining institute, Bhaga; and around ten other colleges. Besides, there are around 500 schools. Court - The court of District and Session Judge is there at Dhanbad. TABLE-1 Details of population, its density, major surface water bodies, tourist spots, schools and the health care units 5 Name of block areas Sadar Jharia Govindpur Nirsa T Area (Sq. Km) 128.82 90.77 334.44 416.85 a Population (2001) 564468 475341 201876 376843 b Densityl 4382 5224 604 904 Majore Surface water Damodar, Damodar, Damodar, Damodar, body Barakar Barakar Barakar Barakar Tourist1 Spot NA NA NA Panchet & Maithan Dam Primary & Middle 155 119 144 215 School Healthcare unit 93 44 4 22 1.6 SourceE -- Govt. -

The Temple Sites at Telkupi
71828_ICOMOS_Markz_6er_Korr4 20.03.2008 14:11 Uhr Seite 88 88 India Heritage at Risk 2006/2007 INDIA of the temples in original condition in 1957 is inadequate and we The Temple Sites at Telkupi (“Bhairavasthan”) have to rely on the photos taken in l872-73, by Beglar; the photos Jaina Architectural Remains Submerged by of Bloch in l903, and those of Bose in l929. This is supplemented Panchet Dam in Jharkhand and West Bengal by the photos of the ASI Eastern Circle taken in 1960. Beglar had documented Temples 2, 3, 4, 5, 6, 7, 8, 12, 15, and l8 in l872-73. We have to rely on his photos and documentation to get a picture of With his report on the consequences of the Panchet Dam, erected the Bhairavasthan temples in their pristine condition. half a century ago, Bulu Imam is complementing his report in The Director General of the ASI visited the Telkupi site with Dr. Heritage at Risk 2004/2005, pp. 94-103 (“Threatened Jaina Mrs. Debala Mitra and they went to Bhairavasthan and according to Heritage Route in Jharkand and West Bengal”). More than 20 tem- Mitra in the Preface to her monograph on Telkupi (1969) they ples from the 8th to 12th centuries (Pala period) were submerged learned locally that most of the temples and the greater part of the between 1956 and 1962 by the waters of the Damodar river. In the village had gone under water and find only the tops of Temples 6, meantime, the remains of these ruined temples are becoming visi- 8, 9, 10, 14, 15 and l6 protruding above the waters of the Damodar, ble again in the silted-up reservoir. -

Jharkhand BSAP
DRAFT REPORT BIODIVERSITY STRATEGY & ACTION PLAN FOR JHARKHAND MANDAR NATURE CLUB ANAND CHIKITSALAYA ROAD, BHAGALPUR, Bihar - 812002 Prepared & Edited by: Arvind Mishra Programme Coordinator Mandar Nature Club Phone: 0641-2423479, Fax- 2300055 (PP) E-mail: [email protected] & [email protected] Coordinating Agency : Mandar Nature Club (MNC) (Regd. Society No. 339/1992-93) Anand Chikitsalaya Road Bhagalpur, Bihar - 812002, India. Phone: 0641-2423479/ 2429663/2300754 Technical Advisors: 1. Dr. Tapan Kr. Ghosh, President, MNC & Reader, University Deptt. of Zoology, T.M.Bhagalpur University, Bhagalpur. 2. Dr. Sunil Agrawal, Secretary, MNC, and a prominent Social worker. 3. Dr. Amita Moitra, Vice President, MNC & Reader, University Deptt. of Zoology, T.M.Bhagalpur University, Bhagalpur. 4. Dr. Tapan Kr. Pan, University Deptt. of Botany, T.M.Bhagalpur University, Bhagalpur. 5. Dr. Gopal Ranjan Dutta, University Deptt. of Zoology, T.M.Bhagalpur University, Bhagalpur. 6. Dr. D.N.Choudhary, P. N. College, Dept. of Zoology, Parsa, Saran, Bihar Compiled by: Dr. Manish Kumar Mishra, Ph.D. (Geography), T.M.Bhagalpur University, Bhagalpur. CONTENTS PAGES INTRODUCTION 5 1. METHODOLOGY 5 2. HISTORY 5 - 6 3. GEOGRAPHY 7 -8 4. PROFILES 8- 20 5. ART & CULTURE 20-22 6. TOURISM IN JHARKHAND 22-25 7. TRADITION, RELIGION & BIODIVERSITY 25-26 8. AGRICULTURE 26-34 9. CENTRAL SPONSORED SCHEMES FOR RURAL DEVELOPMENT 34-36 10. FLORA 36-41 11. FAUNAL BIODIVERSITY 42-45 12. FOREST & WILDLIFE 45-54 13 PROBLEMS 55-64 14. ISSUES 64-71 15. EFFORTS 71-80 16. GAPS 80-82 17. SUGGESTIONS 82-89 18. KEY REFERENCES 90-91 19. ANNEXURE (Avifauna of Jharkhand) ACKNOWLEDGEMENT We express our gratitude to the Kalpvriksha, Biotech Consortium and Ministry of Environment & Forests, Govt. -

Officename Chanda B.O Mirzachowki S.O Boarijore B.O Bahdurchak B.O
pincode officename districtname statename 813208 Chanda B.O Sahibganj JHARKHAND 813208 Mirzachowki S.O Sahibganj JHARKHAND 813208 Boarijore B.O Godda JHARKHAND 813208 Bahdurchak B.O Godda JHARKHAND 813208 Beniadih B.O Godda JHARKHAND 813208 Bhagmara B.O Godda JHARKHAND 813208 Bhagya B.O Godda JHARKHAND 813208 Chapri B.O Godda JHARKHAND 813208 Mandro B.O Sahibganj JHARKHAND 813208 Maniarkajral B.O Godda JHARKHAND 813208 Mordiha B.O Godda JHARKHAND 813208 Rangachak B.O Godda JHARKHAND 813208 Sripurbazar B.O Sahibganj JHARKHAND 813208 Thakurgangti B.O Godda JHARKHAND 814101 Bandarjori S.O Dumka JHARKHAND 814101 S.P.College S.O Dumka JHARKHAND 814101 Dumka H.O Dumka JHARKHAND 814101 Dumka Court S.O Dumka JHARKHAND 814102 Amarapahari B.O Dumka JHARKHAND 814102 Bhaturia B.O Dumka JHARKHAND 814102 Danro B.O Dumka JHARKHAND 814102 Sinduria B.O Dumka JHARKHAND 814102 Ramgarah S.O Dumka JHARKHAND 814102 Gamharia B.O Dumka JHARKHAND 814102 Bandarjora B.O Dumka JHARKHAND 814102 Bariranbahiyar B.O Dumka JHARKHAND 814102 Bhalsumar B.O Dumka JHARKHAND 814102 Chhoti Ranbahiyar B.O Dumka JHARKHAND 814102 Ghaghri B.O Dumka JHARKHAND 814102 Kakni Pathria B.O Dumka JHARKHAND 814102 Khudimerkho B.O Dumka JHARKHAND 814102 Kairasol B.O Godda JHARKHAND 814102 Lakhanpur B.O Dumka JHARKHAND 814102 Mahubana B.O Dumka JHARKHAND 814102 Piprakarudih B.O Dumka JHARKHAND 814102 Sushni B.O Dumka JHARKHAND 814103 Kathikund S.O Dumka JHARKHAND 814103 Saldaha B.O Dumka JHARKHAND 814103 Sarsabad B.O Dumka JHARKHAND 814103 Kalajhar B.O Dumka JHARKHAND 814103 T. Daldali B.O Dumka JHARKHAND 814103 Astajora B.O Dumka JHARKHAND 814103 Pusaldih B.O Dumka JHARKHAND 814103 Amgachi B.O Dumka JHARKHAND 814103 B. -
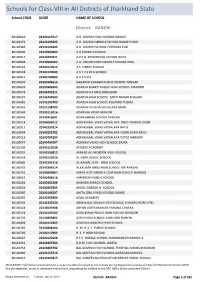
Schools for Class-VIII in All Districts of Jharkhand State School CODE UDISE NAME of SCHOOL
Schools for Class-VIII in All Districts of Jharkhand State School CODE UDISE NAME OF SCHOOL District: RANCHI 80100510 20140117617 A G CHURCH HIGH SCHOOL RANCHI 80100376 20140105605 A G CHURCH MIDDLE SCHOOL KANKE HUSIR 80100383 20140106203 A G CHURCH SCHOOL FURHURA TOLI 80100806 20140903803 A G CHURCH SCHOOL 80100917 20140207821 A P E G RESIDENTIAL SCHOOL RATU 80100808 20140904002 A Q ANSARI URDU MIDDLE SCHOOL IRBA 80100523 20140119912 A S PUBLIC SCHOOL 80100524 20140120009 A S T V S ZILA SCHOOL 80100411 20140109003 A V K S H S 80100299 20140306614 AADARSH GRAMIN PUBLIC SCHOOL TANGAR 80100824 20140906303 ADARSH BHARTI PUBLIC HIGH SCHOOL MANDRO 80100578 20142401811 ADARSH H S MCCLUSKIEGANJ 80100570 20142400503 ADARSH HIGH SCHOOL SANTI NAGAR KHALARI 80100682 20142203709 ADARSH HIGH SCHOOL KOLAMBI TUSMU 80100956 20141108209 ADARSH UCHCHA VIDYALAYA MURI 80100504 20140116916 ADARSHA VIDYA MANDIR 80100846 20140913601 ADARSHHIGH SCHOOL PANCHA 80100214 20140603012 ADIVASI BAL VIKAS VIDYALAYA JINJO THAKUR GAON 80100911 20140207814 ADIVASI BAL VIKAS VIDYALAYA RATU 80100894 20140202702 ADIVASI BAL VIKAS VIDYALAYA TIGRA GURU RATU 80100119 20140704204 ADIVASI BAL VIKAS VIDYALAYA TUTLO NARKOPI 80100647 20140404507 ADIWASI VIKAS HIGH SCHOOL BAJRA 80101106 20140113028 AFAQUE ACADEMY 80100352 20140100813 AHMAD ALI MORDEN HIGH SCHOOL 80100558 20140123620 AL-HERA PUBLIC SCHOOL 80100685 20142203716 AL-KAMAL PLAY HIGH SCHOOL 80100332 20142303514 ALKAUSAR GIRLS HIGH SCHOOL ITKI RANCHI 80100741 20140803807 AMAR JYOTI MIDDLE CUM HIGH SCHOOL HARDAG 80100651 20140404516 -

Download Article (PDF)
CITATION Chakraborty, Rina 2011. Mammalian and Avian Faunal Diversity in Damodar Valley under DVC Project Area, Rec. zool. Surv. India, Occ. Paper No., 328 1-38, (Published by the Director, Zool. Surv. India, Kolkata) Published: May, 2011 ISBN 978-81-8171-289-9 © Gout. of India, 2011 ALL RIGHTS RESERVED • No Part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior permission of the publisher. • This book is sold subject to the condition that it shall not, by way of trade, be lent, resold, hired out or otherwise disposed off without the publisher's consent, in a form of binding or cover other than that in which, it is published. • The correct price of this publication is the price printed on this page. Any revised price indicated by a rubber stamp or by a sticker, or by any other means is incorrect and should be unacceptable. PRICE Indian ~ 150.00 Foreign $ 9 £ 6 Published at the Publication Division, by the Director, Zoological Survey of India, 234/4 A.J.e. Bose Road, 2nd MSO Building, Nizam Palace (13th floor), Kolkata 700 020 and printed at Typographia, Kolkata 700 012. KI!;~~~RENCE NOT FOR ISSUE RECORDS OF THE ZOOLOGICAL SURVEY OF INDIA OCCASIONAL PAPER No. 328 2011 Page 1-38 CONTENTS INTRODUCTION .................................................................................................................. 1 TOPOGRAPHY .................................................................................................................... -

Damodar Valley Corporation
Damodar Valley Corporation Seven Glorious Decades of Corporate Excellence with Social Responsibility : Generation and Distribution of Electricity in Chemical, Pharmaceutical and Agriculture Industries By Dr. Prabir Kumar Mukhopadhyay Member Secretary, DVC 25th May 2019 1 The Beginning Damodar Valley Corporation (DVC) came into being on 7th July, 1948 by an Act (Act No. XIV) of the Constituent Assembly as the first multipurpose river valley project of Independent India on the lines of Tennessee Valley Authority (TVA) of USA. DVC command area is spread over 24,235 sq. kms in Jharkhand & West Bengal. First Multipurpose River Valley Project of Independent India 2 DVC Act - Mandate Economic Development Environmental Social Protection Development - Generation (hydro- electric & thermal), - Flood control - Public health and the transmission & agricultural, industrial, - Afforestation and economic and general distribution of control of soil erosion in electricity. well being in the the Damodar Valley. Damodar Valley - Irrigation & water Supply. 3 DVC Command Area: 24,235 sq. kms KTPS JHARKHAND MPL BPSCL RTPS DSTPS MTPS WEST BENGAL Joint Ventures 4 Turning 2016 First 600 MW units of DVC commissioned at Raghunathpur Points and TPS Milestones Greenfield expansion projects at Durgapur, Koderma & 2009-2014 Raghunathpur. MPL a JV with Tata Power commissioned. 2001 A Joint Venture BPSCL is formed between DVC and SAIL 1981 DVC formally launches its Social Integration Programme as a pioneering effort in the area of CSR. 1963 First reheat unit of India commissioned at Chandrapura TPS. CTPS was the largest plant in Asia. 1953 Commissioning of first dam at Maithon on the river Barakar. Bokaro Thermal was the first pulverised fuel power plant in Asia. -

India Heritage at Risk 2004/2005
Icomos2005 G-N.XP 22.03.2005 11:35 Uhr Seite 94 94 India Heritage at Risk 2004/2005 INDIA Threatened Jaina Heritage Route in Jharkhand and West Bengal Buddhism and Jainism are very old traditions in the old district of Super Thermal Power Projects (STPPs). Also, more than seventy Hazaribagh which included Shikarbhum, Manbhum, Dalbhum and opencast coal mines will destroy over two hundred villages and adjoining East Singhbhum. The Jain heritage was more obvious in the Buddhist heritage of the valley. This includes a rich treasure of the three latter areas, while Buddhism was exclusively of Shikarb- megalith sites, the Mauryan Period Buddhist sites, prehistoric hum (Hazaribagh). The Lord Buddha was worshipped in Haza- rockart sites, and palaeo-archaeological sites. Both UNESCO and ribagh and the Upper Damodar Valley as Mahadeva. We still find ICOMOS are aware of the matter and it has been published in the names associated with Buddhism throughout the district as well as 2001–2002, 2002–2003 Heritage at Risk Reports. the physical presence of Buddhism in Buddhist sacred symbols The mining operations have been in their first stage since 1986 found throughout the sacred village painting traditions of the great and already five mines have started and five more cleared, as well marriage mural art of Khovar, and the harvest mural art of Sohrai, as two big dams and two STTPs. But there is still time if a strong for which Hazaribagh is justly famous. international appeal is mounted. In the Lower Damodar Valley there is the problem of destruc- The Jain and Buddhist heritage of Jharkhand and neighbouring tion of ancient Jain temples in the score from flooding in dams West Bengal has been wilfully destroyed by so-called develop- such as the Panchet Dam on the river Damodar (1969), without ment projects such as big dams and mining in modern India. -
Evaluation of Water Quality Index for Drinking Purposes in the Case of Damodar River, Jharkhand and West Bengal Region, India
ediation & m B re i o o i d B e f g o r a l Journal of Mukherjee et al., J Bioremed Biodeg 2012, 3:9 d a a n t r i o u DOI: 10.4172/2155-6199.1000161 n o J ISSN: 2155-6199 Bioremediation & Biodegradation Research Article OpenOpen Access Access Evaluation of Water Quality Index For Drinking Purposes In The Case of Damodar River, Jharkhand and West Bengal Region, India Diptangshu Mukherjee, S Lata Dora and RK Tiwary* CSIR-Central Institute of Mining & Fuel Research, Council of Scientific & Industrial Research, Barwa Road, Dhanbad-826015, Jharkhand, India Abstract Damodar River is one of the most important fresh water source in Jharkhand and West Bengal. Its water is used for drinking by the growing population and also used in allied Industries situated down the stretch. The water quality is being reducing day by day due to heavy input of sewage and wastes from industrial effluents, cattle stock breeding, irrigation and human activities etc. Therefore, an attempt has been made to assess the quality of water in relation to know the pollution load in terms of biological and various physicochemical properties by evaluation of Quality Index (QI). This technique is very useful tool for quick assessment of any water system. The evaluation of five QI parameters has been taken: pH, TDS (total dissolved solid), BOD5 (biochemical oxygen demand), DO (Dissolved Oxygen) and MPN (Most Probable Number). The study shows that the river water is not potable at maximum sampling stations and are highly fecal contaminated. According to the desirable limit, maximum locations are also not even suitable for taking bath.