Study of Leptospirosis Study of Leptospirosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thematic Area
1 ANNUAL REPORT 2019-20 (April 2019-March 2020) APR SUMMARY Name of the KVK:ICAR Krishi Vigyan Kendra, Ariyalur District, Tamil Nadu 1. Technology Assessment No. of Technology Category No. of Trials No. of Farmers Assessed & Refined Technology Assessed Crops 8 30 30 Livestock --- --- --- Various enterprises --- --- --- Total 8 30 30 Technology Refined Crops --- --- --- Livestock --- --- --- Various enterprises --- --- --- Total --- --- --- Grand Total 8 30 30 2. Frontline demonstrations No. of Details Area (ha) Units/Animals Farmers/Locations Oilseeds 50 20 --- Pulses 35 14 --- Cereals 10 4 --- Millets 5 2 --- Vegetables 25 8.2 --- Fruits 5 2 --- Other crops 5 2 --- Total 135 52.2 --- Livestock & Fisheries 23 --- 20 animals Other enterprises 5 --- 20 farmers/group Total 28 --- Grand Total 163 52.2 3. Training Programmes Total Clientele No. of Courses Male Female participants Farmers & farm women 98 2,050 1038 3,088 Rural youths 17 316 171 487 Extension functionaries 9 255 68 323 Sponsored Training 3 93 30 123 Vocational Training 8 141 34 175 Total 135 2,855 1341 4,196 2 4. Extension Programmes Category No. of Programmes Total Participants Extension activities 446 8,468 Other extension activities 503 Mass Total 949 8,468 + Mass 5. Mobile Advisory Services Message Other Crop Livestock Weather Marketing Awareness Total Type enterprise Text only 20 28 - - 4 33 85 Voice only - - - - - - - Voice & Text - - - - - - - Total 20 28 - - 4 33 85 6. Seed & Planting Material Production Quintal/Number Value Rs. Seed (q) 4.31 2,28,675 Planting material (No.) 2,65,815 9,12,950 Bio-Products (kg) 6,256 3,68,050 Livestock Production (No.) 522 2,50,000 Fishery production (No.) --- --- 7. -

Statistical Tables Contents
STATISTICAL TABLES CONTENTS Page S. NO Contents No. 1. AREA AND POPULATION 1.1. AREA , POPULATION , LITERATE , SC S AND ST S- SEX WISE BY BLOCKS 1 1.2. POPULATION BY BROAD INDUSTRIAL CATEGORIES OF WORKERS . 3 1.3. POPULATION BY RELIGION 5 1.4. POPULATION BY AGE GROUPS 6 1.5. POPULATION OF THE DISTRICT -DECENNIAL GROWTH 7 1.6. SALIENT FEATURES OF 2011 CENSUS . 8 2. CLIMATE AND RAINFALL 2.1. TEMPERATURE 9 2.2. SEASON WISE AND MONTH-WISE NORMAL AND ACTUAL RAINFALL DATA 10 2.3. TIME SERIES DATA OF RAINFALL BY SEASONS . 12 3. AGRICULTURE 3.1. Soil Classification (with illustration by map) 14 3.2. Land Utilization 17 3.3. Area and Production of Crops 19 3.4. Agricultural Machinery and Implements 21 3.5. Number and Area of Operational Holdings 22 3.6. Consumption of Chemical Fertilizers and Pesticides 24 3.7. Regulated Markets 25 3.8. Crop Insurance Scheme 26 3.9. Sericulture 27 4. IRRIGATION 4.1. Sources of Water Supply 28 4.2. Actual Area Irrigated by sources. 29 4.3. Area Irrigated by Crops. 30 4.4. Details of Dams, Tanks, Wells and Bore wells 31 5. ANIMAL HUSBANDRY 5.1. Livestock Population 32 5.2. Veterinary Institutions and Animals treated 33 5.3. No. of Veterinary H ospitals 34 5.4. Diary Development 35 -38 5.5. Poultry Development 39 5.6. FISHERIES DEVELOPMENT AND PRODUCTION 39 6. BANKING AND INSURANCE 6.1. Commercial Banks 40 6.2. Insurance Schemes 41 7. CO -OPERATION 7.1. Co-operative Societies 42 8. -

Analysis of Noise Pollution Using Gis in Trichy District
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056 Volume: 07 Issue: 07 | July 2020 www.irjet.net p-ISSN: 2395-0072 ANALYSIS OF NOISE POLLUTION USING GIS IN TRICHY DISTRICT S. Vanitha1, K. Soundhirarajan2, D. Roopa3, A. Dinesh Kumar 4 1P.G student, Dept. of Civil Engineering, Gnanamani College of Engineering, Namakkal, Tamilnadu, India 2Assistant Professor, Dept. of Civil Engineering, Gnanamani College of Technology, Namakkal, Tamilnadu, India 3Assistant Professor, Dept. of Civil Engineering, Gnanamani College of Engineering, Namakkal, Tamilnadu, India 4Assistant Professor, Dept. of Civil Engineering, Master of Simulation Technology,Thuraiyur, Tamilnadu, India, ---------------------------------------------------------------------***---------------------------------------------------------------------- Abstract - In developing country like India with the vehicle population increasing at a rapid rate, the residents of cities are experiencing severe environmental problems that results from road traffic in particular from automobiles. Fast growing vehicle population in town in the recent years, has resulted in considerable increase in traffic on roads causing alarming noise pollution. The spatial analysis and geo statistical methods of GIS can play an important role to control noise pollution. This paper describes a study that aimed at measuring the noise levels at selected points in Trichy district to generate a noise map over the study area. Key Words: Environmental Problems, Road Traffic, Automobiles, Trichy, Pollution, Noise Map, Road Traffic … 1. INTRODUCTION In developing country like India with the vehicle population increasing at a rapid rate, the residents of cities are experiencing severe environmental problems that results from road traffic in particular from automobiles. Fast growing vehicle population in town in the recent years, has resulted in considerable increase in traffic on roads causing alarming noise pollution. -

Performance Budget 2004-2005
1 PERFORMANCE BUDGET 2007-2008 BACKWARD CLASSES, MOST BACKWARD CLASSES AND MINORITIES WELFARE DEPARTMENT 1.INTRODUCTION Protection of the interests of Backward Classes, Most Backward Classes and Denotified Communities and Minorities has always been an object of prime importance of the Government of Tamil Nadu for their Socio Educational and Economic upliftment. To achieve this end the Government of Tamil Nadu is implementing multifarious welfare schemes. No State can be called as truly advanced without advancement in education. On the basis of this concept utmost importance has always been given to remove the backwardness of the people of this State, by making significant budget allocation for implementation of various educational schemes. Backward Classes/Most Backward Classes/Denotified Communities constitute the major groups among the total population of the State. Religion wise population figures in Tamil Nadu with reference to the 2001 Census are as follows:- 2 (Figures in Lakhs) % of Census year Population RELIGION 1981 1991 2001 % HINDUS 426.52 492.17 549.86 88.11 ISLAMISTS 26.91 31.06 34.7 5.56 CHRISTAINS 29.38 33.9 37.88 6.07 SIKHS 0.1 0.11 0.12 0.02 JAINS 0.63 0.73 0.81 0.13 BUDDISTS 0.05 0.06 0.065 0.01 OTHERS 0.05 0.06 0.065 0.01 NWTO STATE 0.44 0.5 0.56 0.09 Total 484.08 558.59 624.06 100 At Government level, the Secretary is heading the Department of Backward Classes, Most Backward Classes and Minorities Welfare. Different schemes and policies of the Government for the upliftment of the Backward Classes, Most Backward Classes and Denotified Communities and Minorities are implemented by the Director of Backward Classes and Minorities Welfare and Director of Most Backward Classes and Denotified Communities. -

Ii Pullambadi Canal
DEPARTMENT OF ECONOMICS St. JOSEPH’S COLLEGE (Autonomous) (Affiliated to Bharathidasan University, Tiruchirappalli) TIRUCHIRAPPALLI – 620 002. Dr. G. GNANASEKARAN M.A., M.B.A., M.Phil., Ph.D., Head & Research Advisor. CERTIFICATE This is to certify that the thesis entitled “AN ECONOMIC ANALYSIS OF WATER USE EFFICIENCY OF FARMERS IN PULLAMBADI CANAL OF TIRUCHIRAPPALLI AND ARIYALUR DISTRICTS, TAMIL NADU” submitted by Mr. G. IRUTHAYARAJ (Reg. No. 011148 / Ph.D.2 / Economics / F.T. / July 2007) is a bonafide record of research work done by him under my guidance as a full time scholar in the Department of Economics, St. Joseph’s College (Autonomous), Tiruchirappalli and that the thesis has not previously formed the basis for the award to the candidate of any degree or any other similar title. The thesis is the outcome of personal research work done by the candidate under my overall supervision. (G. GNANASEKARAN) Station: Tiruchirappalli Date : DECLARATION I hereby declare that the work embodied in this thesis has been originally carried out by me under the guidance and supervision of Dr. G. GNANASEKARAN , Head and Research Advisor, Department of Economics, St. Joseph’s College (Autonomous), Tiruchirappalli - 620 002. This work has not been submitted either in full or in part for any other degree or diploma at any university. (G. IRUTHAYARAJ) Research Scholar Place: Tiruchirappalli Date : ACKNOWLEDGEMENT I wish to place on record the valuable help rendered by various people to complete this dissertation work. I would like to express my profound sense of gratitude to my research adviser and Best Teacher Awardees Dr. G. Gnanasekaran M.A., M.B.A., M.Phil., Ph.D., Head and Associate Professor of Economics, for his stimulating guidance by spending his valuable time with me in sharpening my thinking and analysis, valuable suggestions and continuous encouragement throughout the study. -
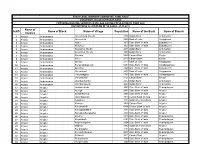
S.NO Name of District Name of Block Name of Village Population Name
STATE LEVEL BANKERS' COMMITTEE, TAMIL NADU CONVENOR: INDIAN OVERSEAS BANK PROVIDING BANKING SERVICES IN VILLAGE HAVING POPULATION OF OVER 2000 DISTRICTWISE ALLOCATION OF VILLAGES -01.11.2011 Name of S.NO Name of Block Name of Village Population Name of the Bank Name of Branch District 1 Ariyalur Andiamadam Anikudichan (South) 2730 Indian Bank Andimadam 2 Ariyalur Andiamadam Athukurichi 5540 Bank of India Alagapuram 3 Ariyalur Andiamadam Ayyur 3619 State Bank of India Edayakurichi 4 Ariyalur Andiamadam Kodukkur 3023 State Bank of India Edayakurichi 5 Ariyalur Andiamadam Koovathur (North) 2491 Indian Bank Andimadam 6 Ariyalur Andiamadam Koovathur (South) 3909 Indian Bank Andimadam 7 Ariyalur Andiamadam Marudur 5520 Canara Bank Elaiyur 8 Ariyalur Andiamadam Melur 2318 Canara Bank Elaiyur 9 Ariyalur Andiamadam Olaiyur 2717 Bank of India Alagapuram 10 Ariyalur Andiamadam Periakrishnapuram 5053 State Bank of India Varadarajanpet 11 Ariyalur Andiamadam Silumbur 2660 State Bank of India Edayakurichi 12 Ariyalur Andiamadam Siluvaicheri 2277 Bank of India Alagapuram 13 Ariyalur Andiamadam Thirukalappur 4785 State Bank of India Varadarajanpet 14 Ariyalur Andiamadam Variyankaval 4125 Canara Bank Elaiyur 15 Ariyalur Andiamadam Vilandai (North) 2012 Indian Bank Andimadam 16 Ariyalur Andiamadam Vilandai (South) 9663 Indian Bank Andimadam 17 Ariyalur Ariyalur Andipattakadu 3083 State Bank of India Reddipalayam 18 Ariyalur Ariyalur Arungal 2868 State Bank of India Ariyalur 19 Ariyalur Ariyalur Edayathankudi 2008 State Bank of India Ariyalur 20 Ariyalur -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2009-11.0 GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2009 [Price : Rs. 39.20 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 49A] CHENNAI, WEDNESDAY, DECEMBER 16, 2009 Margazhi 1, Thiruvalluvar Aandu–2040 Part II—Section 2 (Supplement) NOTIFICATIONS BY GOVERNMENT AGRICULTURE DEPARTMENT Districts, Blocks and Firkas as approved by State Level Co-ordination Committee on Crop Insurance for Kharif, 2009 Secretariat, 16th December 2009. and also requested the Government to notifiy the Seasonalty discipline in the State Gazette for effective implementation Notification of Crops, Districts, Blocks and Firkas for of National Agricultural Insurance Scheme in Tamil Nadu implementation of National Agricultural Insurance during Kharif 2009. The Government accept the Scheme for Kharif, 2009. recommendation of the Commissioner of Agriculture. No. II(2)/AG/690/2009. 2. The Government of Tamil Nadu under National Agricultural Insurance Scheme hereby notify the Crops, [Agriculture - National Agricultural Insurance Scheme - Districts, Blocks and Firkas for implementation of National Notification of Crops, Districts, Blocks and Firkas for Agricultural Insurance Scheme for Kharif 2009 as shown in implementation of National Agricultural Insurance the Annexure I, II and III to this order. Scheme for Kharif, 2009 - Notification - Orders - Issued.] The following Government Order is published:— 3. Further, the claims under National Agricultural Insurance Scheme shall be settled on the basis of yield [G.O. Rt. No. 207, Agriculture (AP1), 20th August 2009.] data furnished by the Director, Economics and Statistics for READ: the notified Crops, Districts, Blocks and Firkas for implementation of National Agricultural Insurance Scheme 1. From the Commissioner of Agriculture letter for Kharif 2009 and not on the basis of Annavari Certificate/ No. -

Protestant Translations of the Bible (1714-1995) and Defining a Protestant Tamil Identity
Protestant Translations of the Bible (1714-1995) and Defining a Protestant Tamil Identity Hephzibah Israel Thesis submitted for the Degree of Doctor of Philosophy to the University of London Department of the Languages and Cultures of South Asia School of Oriental and African Studies March 2004 ProQuest Number: 10673236 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 10673236 Published by ProQuest LLC(2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 Abstract The thesis aims to analyse the construction of a Protestant Tamil identity primarily through the examination of six Protestant translations of the Bible in Tamil and Protestant Tamil poetry. The chapters discuss the points of conflict that arose as a result of the different strategies of assimilation adopted by Protestant missionaries and Protestant Tamils. Chapter 1 has two main sections. The first section provides an outline of the various levels of influence that Catholic and Protestant missionaries had on Tamil language and literature. The second section gives an historical delineation of Protestant translations of the six Tamil Bible versions that the thesis discusses in detail. -

Citizen's Charter
SOCIAL WELFARE AND NUTRITIOUS MEAL PROGRAMME DEPARTMENT CITIZEN’S CHARTER 2019 © GOVERNMENT OF TAMIL NADU 2019 CONTENTS Sl. CHAPTER PAGE No. 1 Introduction 1 2.1 Child Welfare 5 2.2 Women Welfare 10 2.3 Senior Citizens Welfare 37 2.4 Third Gender Welfare 53 2.5 Tamil Nadu State Commission for Women 55 Puratchi Thalaivar M.G.R. Nutritious Meal 3 57 Programme 4 Integrated Child Development Services Scheme 62 5 Social Defence 73 6 Social Security Pension Schemes 132 SOCIAL WELFARE AND NUTRITIOUS MEAL PROGRAMME DEPARTMENT CITIZEN’S CHARTER – 2019-2020 CHAPTER - 1 INTRODUCTION The implementation of the schemes of the welfare of women, children, senior citizens and third gender is done by Social Welfare and Nutritious Meal Programme Department through the Commissionerate of Social Welfare, Commissionerate of Social Defence and the Directorate of Integrated Child Development Services. This Department also functions as nodal agency for various Social Security Pension Schemes implemented through the Commissionerate of Revenue Administration. 2. The Commissionerate of Social Welfare implements welfare schemes like Marriage Assistance Schemes, Girl Child Protection Scheme, Nutritious Meals and four sets of uniforms to the school children. Service Homes for the protection of destitute, deserted women and for young girls rescued from child marriage have been established and the protection of the women and children in distress are taken care of. To provide safety and security to working women who are away from their homes, to stay in a safe environment with amenities at affordable rates, 28 Working Women Hostels are functioning in the State. Training and self-employment of women are thrust areas and 98 Women Tailoring Industrial Co-operative Societies run by the Commissionerate of Social Welfare provide gainful employment for women. -

Sample Collection Centre for COVID
Sample Collection Centers # State District Centre Name Centre Address 1 TAMIL NADU Ariyalur ANDIMADAM MMU PHC ANDIMADAM 2 TAMIL NADU Ariyalur ANDIMADAM PHC ANDIMADAM 3 TAMIL NADU Ariyalur ANGANUR PHC ANGANUR 4 TAMIL NADU Ariyalur ARIYALUR URBAN MMU PHC ARIYALUR 5 TAMIL NADU Ariyalur ARIYALUR URBAN PHC ARIYALUR 6 TAMIL NADU Ariyalur ELAKURICHI PHC ELAKURICHI GOVERNMENT DISTRICT HEADQUATERS ARIYALUR 7 TAMIL NADU Ariyalur HOSPITAL 8 TAMIL NADU Ariyalur GOVT. DISTRICT HQ HOSPITAL ARIYALUR 9 TAMIL NADU Ariyalur JAYAMKONDAM GH JAYAMKONDAM GH 10 TAMIL NADU Ariyalur JAYAMKONDAM GH JAYAMKONDAM 11 TAMIL NADU Ariyalur JAYANKONDAM URBAN PHC JAYANKONDAM 12 TAMIL NADU Ariyalur KADUGUR UGPHC KADUGUR 13 TAMIL NADU Ariyalur KALLANKURICHY PHC KALLANKURICHY 14 TAMIL NADU Ariyalur KEELAPALUR PHC KEELAPALUR 15 TAMIL NADU Ariyalur KULUMUR PHC KULUMUR 16 TAMIL NADU Ariyalur KUMILIYAM ICTC UGPHC KUMILIYAM 17 TAMIL NADU Ariyalur KUMILIYAM UGPHC KUMILIYAM 18 TAMIL NADU Ariyalur KURUVADI MMU PHC GURUVADI 19 TAMIL NADU Ariyalur KURUVADI PHC KURUVADI 20 TAMIL NADU Ariyalur MANAKKAL PHC MANAKKAL 21 TAMIL NADU Ariyalur MANAKUDAIYAN PHC MANAKUDAIYAN .SENDHURAI MANAKUDIAYAN PRIMARY HEALTH CENTRE MANAKUDAIYAN 22 TAMIL NADU Ariyalur 23 TAMIL NADU Ariyalur MARUTHUR PHC MARUTHUR 24 TAMIL NADU Ariyalur MEENSURITI BLOCK PHC ICTC MEENSURITI 25 TAMIL NADU Ariyalur MEENSURITY CHC MEENSURITY JAYAKONDAM 26 TAMIL NADU Ariyalur MEENSURITY UGPHC MEENSURITY 27 TAMIL NADU Ariyalur PONPARAPPI PHC PONPARAPPI POYYATHANALLUR PRIMARY HEALTH CENTRE POYYATHANALLUR 28 TAMIL NADU Ariyalur 29 TAMIL NADU Ariyalur POYYUR PRIMARY HEALTH CENTRE POYYUR 30 TAMIL NADU Ariyalur SENDHURAI GH SENDHURAI 31 TAMIL NADU Ariyalur SUNDAKUDI PHC SUNDAKUDI 32 TAMIL NADU Ariyalur SUTHAMALLI PHC SUTHAMALLI 33 TAMIL NADU Ariyalur T PALUR CHC T PALUR 34 TAMIL NADU Ariyalur T PALUR MMU T PALUR COMMUNITY HEALTH CENTRE 35 TAMIL NADU Ariyalur T POTTAKOLLAI PRIMARY HEALTH CENTRE T POTTAKOLLAI 36 TAMIL NADU Ariyalur T. -
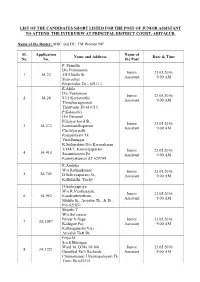
List of the Candidates Short Listed for the Post of Junior Assistant to Attend the Interview at Principal District Court, Ariyalur
LIST OF THE CANDIDATES SHORT LISTED FOR THE POST OF JUNIOR ASSISTANT TO ATTEND THE INTERVIEW AT PRINCIPAL DISTRICT COURT, ARIYALUR. Name of the Roster: MBC and DC, TM Women NP Sl. Application Name of Name and Address Date & Time No. No. the Post P. Sumathi D/o Ponnusamy Junior 23.05.2016, 1 JA 22 3/8 Middle St Assistant 9.00 AM Siruvachur Perambalur Dt – 621113. R.Akila D/o Veeramani Junior 23.05.2016, 2 JA 28 93/1 Keelaveethi Assistant 9.00 AM Thiruthuraipoondi Thiruvarur Dt 614713. P.Kalaiselvi D/o Perumal Pillaiyar kovil St., Junior 23.05.2016, 3 JA 272 Kommandhapuram Assistant 9.00 AM Chettiyar patti Rajapalayam Tk Virudhunagar K.Sutharshini D/o.Karunakaran 3/144/1, Kamarajapuram Junior 23.05.2016, 4 JA 410 Swamithoppu Po Assistant 9.00 AM Kanniyakumari dT 629704 R.Ambika W/o Rathnakumar Junior 23.05.2016, 5 JA 745 D.Subrayapuram St., Assistant 9.00 AM Kallukuzhi, Trichy D.Sathiyapriya W/o.R.Vradharajan, Junior 23.05.2016, 6 JA 962 Kandiratheertham, Assistant 9.00 AM Middle St., Ariyalur Tk., & Dt., Pin-621851 Shanthi.T W/o.Selvarasu Poiyar Village Junior 23.05.2016, 7 JA 1087 Kadugur(Po) Assistant 9.00 AM Kallanguruchi(Via) Ariyalur Tk& Dt. Priya.M S/o.S.Murugan Ward 18, D.No.10/160 Junior 23.05.2016, 8 JA 1221 Gurukkal Palli Backside Assistant 9.00 AM Chinnamanur, Uthamapalayam Tk Theni Dt-625515. Mariswari.P D/o.Pandi.K 24/52, Meenakshi Nagar Junior 23.05.2016, 9 JA 1224 6th West Cross Street Assistant 9.00 AM Meenakshi Nagar, Madurai Dt-625012. -

Ariyalur District - an Outline
DISTRICT STATISTICAL HAND BOOK THERE CANNOT BE A GOOD PLAN FOR ECONOMIC PROGRESS WITHOUT ADEQUATE DATA AND THERE CANNOT BE ADEQUATE DATA WITHOUT A GOOD PLAN FOR COLLECTING THEM . DR.P.C.MAHALANOBIS PREFACE There is an encouraging demand for Block level and District level Statistics for formulating suitable policies by the Government and for the planning purpose at micro level. Maintenance of Block / District level information are assuming importance in the context of planning. An encouraging shift is being made through the introduction of Block level planning to decentralize the planning process. It is to be made obligatory that all the Government agencies operating at Block / District level should spare a copy of the periodical reports to the Block / District Statistical Unit . In the long run these units become a purveyor of information generated in these areas. The District Statistical Hand Book 2018 – 19 is Eleventh in the series. The main purpose of the present effort is to bring out a case for providing information (Data) at Block / District level. The Hand Book is revised to the extent possible. The information furnished in the Hand Book have been collected from various Heads of Departments. The Data furnished in this Book are widely used by needy whether it is an official or research organization. The co-operation extended by the various Departments / Agencies in furnishing the Data is gratefully acknowledged. I also acknowledge the tireless work rented by the Statistical Unit of this District for the successful presentation of the Hand Book. Deputy Director of Statistics, Ariyalur ARIYALUR DISTRICT - AN OUTLINE INTRODUCTION : Ariyalur District came in to existence after bifurcation of Perambalur district with effect from 23.11.2007 as per G.O MS.No 683 Revenue / dept., dated 19.11.2007.