Electrical Conductivity of Fluorite and Fluorine Conduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Heat of Combustion of Beryllium in Fluorine*
JOURNAL OF RESEARCH of the National Bureau of Standards -A. Physics and Chemistry Vol. 73A, No.3, May- June 1969 The Heat of Combustion of Beryllium in Fluorine* K. L. Churney and G. T. Armstrong Institute for Materials Research, National Bureau of Standards, Washington, D.C. 20234 (February 11, 1969) An expe rimental dete rmination of the e ne rgies of combustion in Auorine of polyte traAuoroethylene film and Q.o wder and of mixtures of beryllium with polytetraAuoroethyle ne gi ves for reacti on ( 1)f).H ~.or= - 1022.22 kJ 111 0 1- 1 (- 244.32 kcal mol - I) wit h a n ove ra ll precision of 0.96 kJ 111 0 1- 1 (0. 23 kcal 111 0 1- 1 ) at the 95 pe rce nt confid ence limit s. The tota l un cert a int y is estimated not to exceed ±3.2 kJ mol- I (±0.8 kcal mol - I). The measureme nts on polytetraflu oroeth yle ne giv e for reaction (2a) and reacti on (2 b) f).H ~. o c =- 10 369. 7 and - 10392.4 Jg- I, respective ly. Overall precisions e xpressed at the 95 pe rcent confide nce Ijmits are 3.3 and 6.0 Jg- I, respective ly. Be(c)+ F,(g) = BeF2(a morphous) (1) C,F.(polym e r powd er) + 2F2(g) = 2CF.(g) (2a) C2F.(polyme r film ) + 2F2 (g) = 2CF.(g) (2b) Be2C and Be metal were observed in a small carbonaceous residue from the co mbustion of the beryll iul11 -polytetraAuoroethylene mixtures. -

Abstract Spectroscopic Characterization of Fluorite
ABSTRACT SPECTROSCOPIC CHARACTERIZATION OF FLUORITE: RELATIONSHIPS BETWEEN TRACE ELEMENT ZONING, DEFECTS AND COLOR By Carrie Wright This thesis consists of two separate papers on color in fluorite. In the first paper, synthetic fluorites doped with various REEs (10-300 ppm) were analyzed using direct current plasma spectrometry, optical absorption spectroscopy, fluorescence spectrophotometry, and electron paramagnetic resonance spectroscopy before and after receiving 10-25 Mrad of 60Co gamma irradiation. The combined results of these techniques indicate that the irradiation-induced color of the Y-, Gd-, La- and Ce-doped samples are the result of a REE-associated fluorine vacancy that traps two electrons. Divalent samarium may be the cause of the irradiation-induced green color of the Sm- doped sample. In the second paper, fluorite crystals from Bingham, NM, Long Lake, NY, and Westmoreland, NH were similarly investigated to determine the relationship between sectorally zoned trace elements, defects, and color. The results indicate causes of color similar to those in the synthetic samples with the addition of simple F-centers. SPECTROSCOPIC CHARACTERIZATION OF FLUORITE: RELATIONSHIPS BETWEEN TRACE ELEMENT ZONING, DEFECTS AND COLOR A Thesis Submitted to the Faculty of Miami University In partial fulfillment of The requirements for the degree of Master of Science Department of Geology By Carrie Wright Miami University Oxford, OH 2002 Advisor_____________________ Dr. John Rakovan Reader______________________ Dr. Hailiang Dong TABLE OF CONTENTS Chapter 1: Introduction to the cause of color in fluorite 1 Manuscript 1-Chapter 2 29 “Spectroscopic investigation of lanthanide doped CaF2 crystals: implications for the cause of color” Manuscript 2-Chapter 3 95 “Spectroscopic characterization of fluorite from Bingham, NM, Long Lake, NY and Westmoreland, NH: relationships between trace element zoning, defects and color ii TABLE OF FIGURES Chapter 1 Figures 21 Figure 1a. -

Calcium Fluoride Scale Prevention
Tech Manual Excerpt Water Chemistry and Pretreatment Calcium Fluoride Scale Prevention Calcium Fluoride Fluoride levels in the feedwater of as low as 0.1 mg/L can create a scaling potential if Scale Prevention the calcium concentration is high. The calculation of the scaling potential is analogous to the procedure described in Calcium Sulfate Scale Prevention (Form No. 45-D01553-en) for CaSO4. Calculation 1. Calculate the ionic strength of the concentrate stream (Ic) following the procedure described in Scaling Calculations (Form No. 45-D01551-en): Eq. 1 2. Calculate the ion product (IPc) for CaF2 in the concentrate stream: Eq. 2 where: m 2+ 2+ ( Ca )f = M Ca in feed, mol/L m – – ( F )f = M F in feed, mol/L 3. Compare IPc for CaF2 with the solubility product (Ksp) of CaF2 at the ionic strength of the concentrate stream, Figure 2 /11/. If IPc ≥ Ksp, CaF2 scaling can occur, and adjustment is required. Adjustments for CaF2 Scale Control The adjustments discussed in Calcium Sulfate Scale Prevention (Form No. 45-D01553-en) for CaSO4 scale control apply as well for CaF2 scale control. Page 1 of 3 Form No. 45-D01556-en, Rev. 4 April 2020 Figure 1: Ksp for SrSO4 versus ionic strength /10/ Page 2 of 3 Form No. 45-D01556-en, Rev. 4 April 2020 Figure 2: Ksp for CaF2 versus ionic strength /11/ Excerpt from FilmTec™ Reverse Osmosis Membranes Technical Manual (Form No. 45-D01504-en), Chapter 2, "Water Chemistry and Pretreatment." Have a question? Contact us at: All information set forth herein is for informational purposes only. -

Environmental Impact Assessment and Due Diligence Report (DRAFT)
Environmental Impact Assessment and Due Diligence Report (DRAFT) Project Number: 47051-002 May 2015 PRC: Chemical Industry Energy Efficiency and Emissions Reduction Project Prepared by ChemChina and CHC for the Asian Development Bank. This is a version of the draft posted in May 2015 available on http://www.adb.org/Documents/RRPs/?id=47051-002-3. CURRENCY EQUIVALENTS (as of 11 May 2015) Currency unit – yuan (CNY) CNY1.00 = $ 0.1633 $1.00 = CNY 6.1254 ABBREVIATIONS ADB – Asian Development Bank AP – Affected Person API – American Petroleum Institute ASL – Above Sea Level CCPS – Center for Chemical Process Safety CEMS – Continuous Emissions Monitoring System ChemChina – China National Chemical Corporation CHC – China Haohua Company CHP – Combined Heat and Power CNY – Chinese Yuan CSEMP – Construction Site Environmental Management Plan DCS – Distributed Control System EA – Executing Agency EHS – Environment, Health and Safety EHSS – Environment, Health and Safety Specialist EHSU – Environment, Health and Safety Unit EIA – Environmental Impact Assessment EMoP – Environmental Monitoring Plan EMP – Environmental Management Plan EMS – Environmental Monitoring Station EPB – Environmental Protection Bureau FSR – Feasibility Study Report Flor – Calcium fluoride (CaF2) or “fluorite” GDP – Gross Domestic Product GHG – Greenhouse Gas GIP – Good International Practice GRM – Grievance Redress Mechanism HDP – High Density Polyethylene IA – Implementing Agency IEE – Initial Environmental Examination IT – Interim Target LIC – Loan Implementation Consultant -
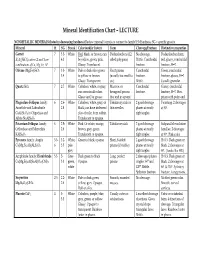
Mineral Identification Chart – LECTURE
Mineral Identification Chart – LECTURE NONMETALLIC MINERALS (listed in decreasing hardness) Review mineral formula to connect to family! H=Hardness; SG = specific gravity Mineral H SG Streak Color (and/or luster) Form Cleavage/Fracture Distinctive properties Garnet 7 3.5- White Red, black, or brown; can Dodecahedrons (12- No cleavage. Dodecahedron form, X3Y2(SiO4)3 where X and Y are 4.3 be yellow, green, pink. sided polygons) Brittle. Conchoidal red, glassy, conchoidal combinations of Ca, Mg, Fe, Al Glassy. Translucent. fracture. fracture, H=7. Olivine (Mg,Fe)2SiO4 7 3.3- White Pale or dark olive green Short prisms Conchoidal Green, conchoidal 3.4 to yellow or brown. (usually too small to fracture. fracture, glassy, H=7. Glassy. Transparent. see). Brittle. Usually granular. Quartz SiO2 7 2.7 White Colorless, white, or gray; Massive; or Conchoidal Glassy, conchoidal can occur in all colors. hexagonal prisms fracture. fracture, H=7. Hex. Glassy and/or greasy. that end in a point. prism with point end. Plagioclase Feldspar family: 6 2.6- White Colorless, white, gray, or Tabular crystals or 2 good cleavage Twinning. 2 cleavages Anorthite and Labradorite 2.8 black; can have iridescent thin needles planes at nearly at 90°. CaAl2Si2O8 to Oligoclase and play of color from within. right angles. Albite NaAlSi3O8 Translucent to opaque. Potassium Feldspar family: 6 2.5- White Pink. Or white, orange, Tabular crystals 2 good cleavage Subparallel exsolution Orthoclase and Microcline 2.6 brown, gray, green. planes at nearly lamellae. 2 cleavages KAlSi3O8 Translucent to opaque. right angles. at 90°. Pink color. Pyroxene family: Augite 5.5- 3.2- White, Green to black; opaque. -

Cleaning Optics
GUIDE TO CLEANING OPTICS Crystal optics are more delicate than glass and should be treated carefully if they have to be cleaned. Some materials are very delicate, and should be treated with extra care. We describe the method, but the technique comes with practice. Handle optics by the edge and use lint-free nylon gloves. Use plastic protective gloves when cleaning with solvent but check that the solvent does not attack the glove. Clean optics as little as possible and only if absolutely necessary. Cleaning may create fine scratches which you cannot see easily but which may contribute to scattering, particularly in the UV. If the optic is mounted, try never to let solvent creep into the mounting ring. Use an air jet and no solvent at all for removal of dust. Very Delicate Materials: CsI, KRS5, Germanium, Zinc Selenide, Zinc Sulphide. These materials are soft, or are inclined to take marks (sleeks) or show up marks easily. They should be washed in a solvent such as methanol or propanol for light marking. Use an environmental friendly solvent such as NuSol Rapide (a replacement for trichloroethylene which is available in the UK) or otherwise acetone, for greasy or waxy contaminants. Soak the optic and wipe while wet with cotton wool (known as absorbent cotton in the USA) dipped in the solvent and let the optic dry by evaporation or assist it with airflow as it is wiped. Delicate Materials: Calcium Fluoride, Coated Materials, Magnesium Fluoride, Other fluorides, Silicon. These should be treated as above where practical, but it is often preferable to clean them carefully with a damp tissue. -

Winter 1998 Gems & Gemology
WINTER 1998 VOLUME 34 NO. 4 TABLE OF CONTENTS 243 LETTERS FEATURE ARTICLES 246 Characterizing Natural-Color Type IIb Blue Diamonds John M. King, Thomas M. Moses, James E. Shigley, Christopher M. Welbourn, Simon C. Lawson, and Martin Cooper pg. 247 270 Fingerprinting of Two Diamonds Cut from the Same Rough Ichiro Sunagawa, Toshikazu Yasuda, and Hideaki Fukushima NOTES AND NEW TECHNIQUES 281 Barite Inclusions in Fluorite John I. Koivula and Shane Elen pg. 271 REGULAR FEATURES 284 Gem Trade Lab Notes 290 Gem News 303 Book Reviews 306 Gemological Abstracts 314 1998 Index pg. 281 pg. 298 ABOUT THE COVER: Blue diamonds are among the rarest and most highly valued of gemstones. The lead article in this issue examines the history, sources, and gemological characteristics of these diamonds, as well as their distinctive color appearance. Rela- tionships between their color, clarity, and other properties were derived from hundreds of samples—including such famous blue diamonds as the Hope and the Blue Heart (or Unzue Blue)—that were studied at the GIA Gem Trade Laboratory over the past several years. The diamonds shown here range from 0.69 to 2.03 ct. Photo © Harold & Erica Van Pelt––Photographers, Los Angeles, California. Color separations for Gems & Gemology are by Pacific Color, Carlsbad, California. Printing is by Fry Communications, Inc., Mechanicsburg, Pennsylvania. © 1998 Gemological Institute of America All rights reserved. ISSN 0016-626X GIA “Cut” Report Flawed? The long-awaited GIA report on the ray-tracing analysis of round brilliant diamonds appeared in the Fall 1998 Gems & Gemology (“Modeling the Appearance of the Round Brilliant Cut Diamond: An Analysis of Brilliance,” by T. -

Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H
Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H. Anderson Report of Investigations 8 doi.org/10.13023/kgs.ri08.13 Series XIII, 2019 Cover Photo: Various alkaline ultramafic rocks showing porphyritic, brecciated, and aphanitic textures, in contact with host limestone and altered dike texture. From left to right: • Davidson North dike, Davidson core, YH-04, 800 ft depth. Lamprophyre with calcite veins, containing abundant rutile. • Coefield area, Billiton Minner core BMN 3. Intrusive breccia with lamprophyric (al- nöite) matrix. • Maple Lake area, core ML-1, 416 ft depth. Lamprophyre intrusive. • Maple Lake area, core ML-2, 513 ft depth. Lamprophyre (bottom) in contact with host limestone (top). Kentucky Geological Survey University of Kentucky, Lexington Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H. Anderson Report of Investigations 8 doi.org/10.13023/kgs.ri08.13 Series XIII, 2019 Our Mission The Kentucky Geological Survey is a state-supported research center and public resource within the University of Kentucky. Our mission is to sup- port sustainable prosperity of the commonwealth, the vitality of its flagship university, and the welfare of its people. We do this by conducting research and providing unbiased information about geologic resources, environmen- tal issues, and natural hazards affecting Kentucky. Earth Resources—Our Common Wealth www.uky.edu/kgs © 2019 University of Kentucky For further information contact: Technology Transfer Officer Kentucky Geological Survey 228 Mining and Mineral Resources Building University of Kentucky Lexington, KY 40506-0107 Technical Level General Intermediate Technical Statement of Benefit to Kentucky Rare earth elements are used in many applications in modern society, from cellphones to smart weapons systems. -

Andradite in Andradite Unusual Growth Zoning in Beryl
Editor Nathan Renfro Contributing Editors Elise A. Skalwold and John I. Koivula Andradite in Andradite ity, but size was not what made it special. As shown in fig- Recently we had the opportunity to examine a dramatic ure 1, close examination of one of the polished crystal faces iridescent andradite fashioned by Falk Burger (Hard Works, revealed a bright reddish orange “hot spot” in the center, Tucson, Arizona) from a crystal originating from the caused by an iridescent inclusion of andradite with a dif- Tenkawa area of Nara Prefecture in Japan. Known as “rain- ferent crystallographic orientation than its host. As seen bow” andradite, this material was previously reported in in figure 2, the inclusion’s different orientation caused the iridescence of the rhomb-shaped “hot spot” to appear and Gems & Gemology (T. Hainschwang and F. Notari, “The cause of iridescence in rainbow andradite from Nara, disappear as the light source was passed over the crystal’s Japan,” Winter 2006, pp. 248–258). The specimen was surface. To see the iridescence from both the host and in- unique for its genesis and optical phenomenon. clusion at the same time, two light sources from opposite Weighing 16.79 ct and measuring 15.41 × 13.86 × 10.49 directions must be used due to the different crystallo- mm, the andradite was very large for its species and local- graphic orientation of the host and inclusion. This elusive optical phenomenon made this Japanese andradite crystal extremely interesting for any aspiring inclusionist. John I. Koivula Figure 1. This 16.79 ct Japanese andradite garnet GIA, Carlsbad exhibits a very unusual rhomb-shaped “hot spot” below the surface of one crystal face. -

Low Temperature Aqueous Solubility of Fluorite at Temperatures of 5, 25, and 50 Oc and Ionic Strengths up to 0.72M
LOW TEMPERATURE AQUEOUS SOLUBILITY OF FLUORITE AT TEMPERATURES OF 5, 25, AND 50 OC AND IONIC STRENGTHS UP TO 0.72M by Richard L. Henry A thesis submitted to the Faculty and Board of Trustees of the Colorado School of Mines in partial fulfillment of the requirement for the degree of Doctor of Philosophy (Geochemistry) Golden, Colorado Date __________________________ Signed: ______________________________ Richard L. Henry Signed: ______________________________ Dr. Wendy J. Harrison Thesis Advisor Golden, Colorado Date __________________________ Signed: ______________________________ Dr. M. Stephen Enders Professor and Head Department of Geology and Geological Engineering ii ABSTRACT Historical fluorite solubility research found that fluorite solubilities varied over three orders-of-magnitude and appear to be inconsistent with several recently determined fluorite solubilities. This research involved a comprehensive series of solubility experiments run over a period of three years using nine natural fluorites and one synthetic fluorite to determine their solubility in low temperature (5, 25, and 50oC) aqueous solutions and ionic strengths ranging from near zero to 0.72M. Equilibrium was approached from both undersaturated and supersaturated initial conditions. The fluorites were chemically and physically characterized using laboratory analyses, thin section petrography, UV fluorescence, and x-ray diffraction. The fluorite samples were generally found to have similar chemical and physical properties. The chemical composition was approximately stoichiometric, with a mean calcium concentration of 51.6 ± 0.6 wt % and mean fluorine concentration of 50.2 ± 0.7 wt %. Fluorite unit cell edge lengths, volumes, and densities averaged 5.4630 ± 0.0007 Å, 163.04 ± 0.06 Å3, and 3.181 ± 0.009 g/cm3, respectively. -

The Eyes of Africa
The Eyes of Africa Where is it from? Erongo Mountain, Erongo Region, Namibia What are its dimensions? H: 22.8 in W: 13.3 in D: 10.2 in How much does it weight? 64.3 Pounds (29.2 kilos) What is this mineral made of? Fluorite & Quartz What is Fluorite (CaF2)? Named in 1797 by Carlo Antonio Galeani Napione from the Latin, fluere = “to flow” (for its use as a flux). The term fluores- cence is derived from fluorite, which will often markedly exhib- it this effect. The element fluorine also derives its name from fluorite. What is Quartz (SiO2)? Quartz has been known and appreciated since prehistoric times. Kristallos is the most ancient name known for quartz, recorded by Theophrastus in about 300-325 BCE. The root words κρύοσ (ice cold) and στέλλειυ (to contract) suggest the ancient belief that kristallos was permanently solidified ice. Brief Description: This specimen is known as “The Eyes of Africa.” It is composed of several white, semi-translucent quartz crystals and large, green and black Alien Eye Fluorites. It was recovered from the Erongo Region of Namibia in 2007. Alien Eyes are a unique and unusual subset of fluorite that differ- entiates itself with its vivid green color and black outer zones that create a diamond shape at each crystal’s center. They also have a naturally formed, complex crystal habit in the form of cuboctahedra. With light, Alien Eye fluorites glow with an incredible otherworldly quality which was what inspired their name. The total number of Alien Eye Fluorites recovered from the find is low, amounting to less than 30 fine specimens, due to the small pocket size and the fact that there was only one single discovery. -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide FIRST EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 1st Edition © 2019 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations.