Transmission of Hepatitis E Virus in Developing Countries
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Giardia Intestinalis, Also Known As G
For Vets General Information Giardiosis is a disease caused by the protozoan parasite Giardia intestinalis, also known as G. duodenalis or G. lamblia. It is the most commonly identified pathogen in outbreaks of waterborne human disease in the USA. Clinical disease typically manifests as diarrhea. By far the most common source of infection for people is surface water contaminated by human fecal material. Within G. intestinalis there are a number of genotypes which are grouped into “assemblages” A to F. Assemblages A and B are capable of infecting several animal species, as well as humans. Other assemblages occur only in animals. Giardia, including zoonotic and species-specific assemblages, occurs frequently in dogs, and less commonly in cats, but the parasite can infect many species including beavers, livestock, ferrets, guinea pigs, gerbils, rats and chinchillas. The risk of zoonotic transmission of Giardia from pets remains controversial and unquantified, but the potential certainly exists. It is therefore important to be aware of the potential risk and encourage pet owners and people who handle animals to take steps to help prevent the spread of Giardia. Prevalence and Risk Factors Humans Giardiosis is endemic worldwide. In industrialized countries the prevalence of Giardia in people is estimated to be between 1-7%, but it may be as high as 50% in developing countries. All age groups are equally affected during epidemics, but both subclinical infection and clinical disease are more common in children in endemic areas. Outbreaks occur regularly in childcare facilities. Immunocompromised individuals are also more commonly affected than members of the general population. -

Globalization and Infectious Diseases: a Review of the Linkages
TDR/STR/SEB/ST/04.2 SPECIAL TOPICS NO.3 Globalization and infectious diseases: A review of the linkages Social, Economic and Behavioural (SEB) Research UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) The "Special Topics in Social, Economic and Behavioural (SEB) Research" series are peer-reviewed publications commissioned by the TDR Steering Committee for Social, Economic and Behavioural Research. For further information please contact: Dr Johannes Sommerfeld Manager Steering Committee for Social, Economic and Behavioural Research (SEB) UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) World Health Organization 20, Avenue Appia CH-1211 Geneva 27 Switzerland E-mail: [email protected] TDR/STR/SEB/ST/04.2 Globalization and infectious diseases: A review of the linkages Lance Saker,1 MSc MRCP Kelley Lee,1 MPA, MA, D.Phil. Barbara Cannito,1 MSc Anna Gilmore,2 MBBS, DTM&H, MSc, MFPHM Diarmid Campbell-Lendrum,1 D.Phil. 1 Centre on Global Change and Health London School of Hygiene & Tropical Medicine Keppel Street, London WC1E 7HT, UK 2 European Centre on Health of Societies in Transition (ECOHOST) London School of Hygiene & Tropical Medicine Keppel Street, London WC1E 7HT, UK TDR/STR/SEB/ST/04.2 Copyright © World Health Organization on behalf of the Special Programme for Research and Training in Tropical Diseases 2004 All rights reserved. The use of content from this health information product for all non-commercial education, training and information purposes is encouraged, including translation, quotation and reproduction, in any medium, but the content must not be changed and full acknowledgement of the source must be clearly stated. -
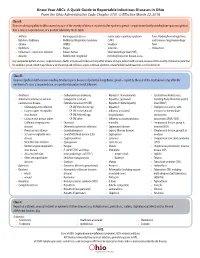
The Know Your Abcs: a Quick Guide to Reportable Infectious Diseases
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective March 22, 2018 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Meningococcal disease • Severe acute respiratory syndrome fever, Marburg hemorrhagic fever, • Botulism, foodborne • Middle East Respiratory Syndrome (SARS) and Crimean-Congo hemorrhagic • Cholera (MERS) • Smallpox fever • Diphtheria • Plague • Tularemia • Yellow fever • Influenza A – novel virus infection • Rabies, human • Viral hemorrhagic fever (VHF), • Measles • Rubella (not congenital) including Ebola virus disease, Lassa Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis C (non-perinatal) • Spotted Fever Rickettsiosis, • Arboviral neuroinvasive and non- carbapenem-resistant • Hepatitis C (perinatal) including Rocky Mountain spotted neuroinvasive disease: Enterobacteriaceae (CP-CRE) • Hepatitis D (delta hepatitis) fever (RMSF) • Chikungunya virus infection • CP-CRE Enterobacter spp. • Hepatitis E • Staphylococcus aureus, with • Eastern equine encephalitis • CP-CRE Escherichia coli • Influenza-associated resistance or intermediate virus disease • CP-CRE Klebsiella spp. -

Reportable Diseases and Conditions
KINGS COUNTY DEPARTMENT of PUBLIC HEALTH 330 CAMPUS DRIVE, HANFORD, CA 93230 REPORTABLE DISEASES AND CONDITIONS Title 17, California Code of Regulations, §2500, requires that known or suspected cases of any of the diseases or conditions listed below are to be reported to the local health jurisdiction within the specified time frame: REPORT IMMEDIATELY BY PHONE During Business Hours: (559) 852-2579 After Hours: (559) 852-2720 for Immediate Reportable Disease and Conditions Anthrax Escherichia coli: Shiga Toxin producing (STEC), Rabies (Specify Human or Animal) Botulism (Specify Infant, Foodborne, Wound, Other) including E. coli O157:H7 Scrombroid Fish Poisoning Brucellosis, Human Flavivirus Infection of Undetermined Species Shiga Toxin (Detected in Feces) Cholera Foodborne Disease (2 or More Cases) Smallpox (Variola) Ciguatera Fish Poisoning Hemolytic Uremic Syndrome Tularemia, human Dengue Virus Infection Influenza, Novel Strains, Human Viral Hemorrhagic Fever (Crimean-Congo, Ebola, Diphtheria Measles (Rubeola) Lassa, and Marburg Viruses) Domonic Acid Poisoning (Amnesic Shellfish Meningococcal Infections Yellow Fever Poisoning) Novel Virus Infection with Pandemic Potential Zika Virus Infection Paralytic Shellfish Poisoning Plague (Specify Human or Animal) Immediately report the occurrence of any unusual disease OR outbreaks of any disease. REPORT BY PHONE, FAX, MAIL WITHIN ONE (1) WORKING DAY Phone: (559) 852-2579 Fax: (559) 589-0482 Mail: 330 Campus Drive, Hanford 93230 Conditions may also be reported electronically via the California -

Know Your Abcs: a Quick Guide to Reportable Infectious Diseases in Ohio
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective August 1, 2019 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Measles • Rubella (not congenital) • Viral hemorrhagic fever • Botulism, foodborne • Meningococcal disease • Severe acute respiratory (VHF), including Ebola virus • Cholera • Middle East Respiratory syndrome (SARS) disease, Lassa fever, Marburg • Diphtheria Syndrome (MERS) • Smallpox hemorrhagic fever, and • Influenza A – novel virus • Plague • Tularemia Crimean-Congo hemorrhagic infection • Rabies, human fever Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis B (perinatal) • Salmonellosis • Arboviral neuroinvasive and carbapenem-resistant • Hepatitis C (non-perinatal) • Shigellosis -

Optimal Vaccine Subsidies for Endemic and Epidemic Diseases Matthew Goodkin-Gold, Michael Kremer, Christopher M
WORKING PAPER · NO. 2020-162 Optimal Vaccine Subsidies for Endemic and Epidemic Diseases Matthew Goodkin-Gold, Michael Kremer, Christopher M. Snyder, and Heidi L. Williams NOVEMBER 2020 5757 S. University Ave. Chicago, IL 60637 Main: 773.702.5599 bfi.uchicago.edu OPTIMAL VACCINE SUBSIDIES FOR ENDEMIC AND EPIDEMIC DISEASES Matthew Goodkin-Gold Michael Kremer Christopher M. Snyder Heidi L. Williams The authors are grateful for helpful comments from Witold Więcek and seminar participants in the Harvard Economics Department, Yale School of Medicine, the “Infectious Diseases in Poor Countries and the Social Sciences” conference at Cornell University, the DIMACS “Game Theoretic Approaches to Epidemiology and Ecology” workshop at Rutgers University, the “Economics of the Pharmaceutical Industry” roundtable at the Federal Trade Commission’s Bureau of Economics, the U.S. National Institutes of Health “Models of Infectious Disease Agent” study group at the Hutchinson Cancer Research Center in Seattle, the American Economic Association “Economics of Infectious Disease” session, and the Health and Pandemics (HELP!) Economics Working Group “Covid-19 and Vaccines” workshop. Maya Durvasula, Nishi Jain, Amrita Misha, Frank Schilbach, and Alfian Tjandra provided excellent research assistance. Williams gratefully acknowledges financial support from NIA grant number T32- AG000186 to the NBER. © 2020 by Matthew Goodkin-Gold, Michael Kremer, Christopher M. Snyder, and Heidi L. Williams. All rights reserved. Short sections of text, not to exceed two paragraphs, may be quoted without explicit permission provided that full credit, including © notice, is given to the source. Optimal Vaccine Subsidies for Endemic and Epidemic Diseases Matthew Goodkin-Gold, Michael Kremer, Christopher M. Snyder, and Heidi L. -

Potential Biomarkers As an Indicator of Vertical Transmission of Johne's
Original Article J Vet Sci 2017, 18(S1), 343-349ㆍhttps://doi.org/10.4142/jvs.2017.18.S1.343 JVS Potential biomarkers as an indicator of vertical transmission of Johne’s disease in a Korean native cattle farm Hong-Tae Park1,†, Hyun-Eui Park1,†, Yong-Il Cho2, Eui-Hyung Kim3, Myunghwan Jung1, Seung Won Shin1, Su-Hyung Lee4, Dae-Yong Kim4, Han Sang Yoo1,* Departments of 1Infectious Diseases and 4Veterinary Pathology, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea 2Department of Animal Science & Technology, Suncheon National University, Suncheon 57922, Korea 3National Institute of Animal Science, Rural Development Administration, Pyeongchang 25340, Korea Paratuberculosis (PTB) is caused by Mycobacterium avium subsp. paratuberculosis (MAP) and is one of the most widespread and economically important diseases in cattle. After birth, calves are raised with natural breast feeding without separation from their mothers in most Korean native cattle (Hanwoo breed) farms. Vertical transmission of PTB has been reported, but the exact PTB infection route has not been revealed in Hanwoo farms. Calves of MAP seropositive dams were tested for MAP presence and MAP antibodies in feces and tissues. MAP was detected in calf tissues by using polymerase chain reaction. Expressions of genes reported to be prognostic biomarkers of MAP infection changed in both calves and cows (p < 0.05). Expression of two genes (HGF and SERPINE1) were significantly decreased in MAP-infected cattle and their offspring (p < 0.01). The results suggest that biomarker gene expression profiles can be useful in detecting early stage MAP infection. Based on the results, complete eradication of MAP may be possible if accurate diagnostic methods to detect infected calves are added to the current PTB eradication strategy, which, because infected individuals are likely to develop into fecal MAP shedders at any time, includes isolation of new born calves and feeding sterilized colostrum. -

Some Simple Rules for Estimating Reproduction Numbers in the Presence of Reservoir Exposure Or Imported Cases
Some simple rules for estimating reproduction numbers in the presence of reservoir exposure or imported cases Angus McLure1*, Kathryn Glass1 1 Research School of Population Health, Australian National University, 62 Mills Rd, Acton, 0200, ACT, Australia * Corresponding author: [email protected] 1 Abstract The basic reproduction number () is a threshold parameter for disease extinction or survival in isolated populations. However no human population is fully isolated from other human or animal populations. We use compartmental models to derive simple rules for the basic reproduction number for populations with local person‐to‐person transmission and exposure from some other source: either a reservoir exposure or imported cases. We introduce the idea of a reservoir‐driven or importation‐driven disease: diseases that would become extinct in the population of interest without reservoir exposure or imported cases (since 1, but nevertheless may be sufficiently transmissible that many or most infections are acquired from humans in that population. We show that in the simplest case, 1 if and only if the proportion of infections acquired from the external source exceeds the disease prevalence and explore how population heterogeneity and the interactions of multiple strains affect this rule. We apply these rules in two cases studies of Clostridium difficile infection and colonisation: C. difficile in the hospital setting accounting for imported cases, and C. difficile in the general human population accounting for exposure to animal reservoirs. We demonstrate that even the hospital‐adapted, highly‐transmissible NAP1/RT027 strain of C. difficile had a reproduction number <1 in a landmark study of hospitalised patients and therefore was sustained by colonised and infected admissions to the study hospital. -

Asymptomatic Subclinical Cases of Coronavirus Disease 2019 Without Viral Transmission in Three Independent Families
Infection and Drug Resistance Dovepress open access to scientific and medical research Open Access Full Text Article SHORT REPORT Asymptomatic Subclinical Cases of Coronavirus Disease 2019 without Viral Transmission in Three Independent Families This article was published in the following Dove Press journal: Infection and Drug Resistance Xian Zhang1,* Purpose: There is increasing evidence indicating that considerable fractions of cases of Liting Chen2,* SARS-CoV-2 infection are asymptomatic. We traced three asymptomatic clusters to inves Jia Wei2 tigate the infectivity of subclinical cases of coronavirus disease 2019 (COVID-19). Jianfeng Zhou2 Patients and Methods: Three medical staff who were asymptomatic were diagnosed with Yang Cao2 coronavirus disease 2019 by serological tests. Their close contacts were systematically evaluated based on COVID-19-related symptoms, nucleic acid tests, serological tests, and Gaoxiang Wang2 chest computed tomography (CT) as needed to determine if they were infected by SARS- 1Department of Ophthalmology, Tongji CoV-2. Hospital, Tongji Medical College, Huazhong University of Science and Results: None of the staff’s close contacts, including 10 family members, were infected by Technology, Wuhan, Hubei 430030, the indexes, even though no protective measures were taken. 2 People’s Republic of China; Department Conclusion: The infectivity of asymptomatic subclinical infection patients of coronavirus of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of disease 2019 seems to be low. Science and Technology, Wuhan, Hubei Keywords: SARS-CoV-2, COVID-19, asymptomatic, close contact, infectivity 430030, People’s Republic of China *These authors contributed equally to this work Introduction Toward the end of 2019, there was an outbreak of severe acute respiratory syn drome coronavirus 2 (SARS-CoV-2). -

Subclinical Avian Influenza A(H5N1) Virus Infection in Human, Vietnam
DISPATCHES leading to hospital admission. Despite intensive care and Subclinical Avian treatment with oseltamivir and antibiotics, the disease pro- gressed, and he died 2 days later. Influenza A(H5N1) A throat swab taken from the index case-patient on day 3 of illness was tested by reverse transcription PCR, and Virus Infection in results were positive for influenza A(H5N1) virus. Hemag- Human, Vietnam glutination inhibition (HI) and microneutralization (MN) tests for H5N1-specific antibodies were negative in sam- Mai Quynh Le, Peter Horby, Annette Fox, ples taken during the acute phase of illness (online Techni- Hien Tran Nguyen, Hang Khanh Le Nguyen, cal Appendix, wwwnc.cdc.gov/EID/article/19/10/13-0730- Phuong Mai Vu Hoang, Khanh Cong Nguyen, Techapp1.pdf). Menno D. de Jong, Rienk E. Jeeninga, On day 5 of illness of the index case-patient, a contact H. Rogier van Doorn, Jeremy Farrar, investigation was initiated. Throat swab specimens were and Heiman F.L. Wertheim collected from 4 household members and 1 close contact of the index case-patient: his spouse (age 47 years), daughter Laboratory-confirmed cases of subclinical infection (age 18 years), daughter-in-law (age 25 years), and grand- with avian influenza A(H5N1) virus in humans are rare, and son (age 1 year) and an unrelated man (age 43 years). None the true number of these cases is unknown. We describe of the contacts had signs or symptoms. Infection control the identification of a laboratory-confirmed subclinical case measures were initiated, and all household members were in a woman during an influenza A(H5N1) contact investiga- tion in northern Vietnam. -

Management of Water-Related Microbial Diseases
Management of Water-related Microbial Diseases First Edition 2003 The Department of Water U Affairs and Forestry Tl I75/DJ Water Research Commission REPORTS IN THIS SERIES This guide forms part of a series which is aimed at water supply agencies, water resource managers, workers in health-related fields, as well as communities throughout South Africa. The guide is intended to provide awareness-building information to keep water supplies clean of microbial contamination and thus reduce the incidence of water-related diseases. The publication of this report emanates from a WRC consultancy no 431: Guide on water-related microbiological diseases. The following documents form part of this series of Guides on the Management of Water-related Microbial Diseases: Vol: 1 What is the problem? - Disease Characteristics. Vol: 2 What causes the problem;1 - A What to Do for Water Suppliers following Diarrhoea Incidents. ' Vol: 3 How great is the problem? - Health Impact Assessment.1 Vol: 4 How dangerous is the problem? - Communicating the Risk.' Vol: 5 What we and our children need to know - Health & Hygiene Awareness.1 Still in preparation. This guide is available from: Director: Institute for Water Quality Studies Water Research Commission Department of Water Affairs & Forestry Private Bag X03 Private Bag X313 Geztna Pretoria 0031 0001 South Africa Tel: 012 808 0374 Tel: 012 330 0340 Fax: 012 808 0338 Fax: 012 331 2565 Disclaimer This report has been reviewed by the Water Research Commission (WRC) and approved for 1 publication. Approval does not signify that the contents necessarily reflect the views and policies of the WRC, nor does mention of trade names or commercial products constitute endorsement or f recommendation for use. -

A New Twenty-First Century Science for Effective Epidemic Response
Review A new twenty-first century science for effective epidemic response https://doi.org/10.1038/s41586-019-1717-y Juliet Bedford1, Jeremy Farrar2*, Chikwe Ihekweazu3, Gagandeep Kang4, Marion Koopmans5 & John Nkengasong6 Received: 10 June 2019 Accepted: 24 September 2019 With rapidly changing ecology, urbanization, climate change, increased travel and Published online: 6 November 2019 fragile public health systems, epidemics will become more frequent, more complex and harder to prevent and contain. Here we argue that our concept of epidemics must evolve from crisis response during discrete outbreaks to an integrated cycle of preparation, response and recovery. This is an opportunity to combine knowledge and skills from all over the world—especially at-risk and afected communities. Many disciplines need to be integrated, including not only epidemiology but also social sciences, research and development, diplomacy, logistics and crisis management. This requires a new approach to training tomorrow’s leaders in epidemic prevention and response. connected, high-density urban areas (particularly relevant to Ebola, Anniversary dengue, influenza and severe acute respiratory syndrome-related coro- collection: navirus SARS-CoV). These factors and effects combine and interact, go.nature.com/ fuelling more-complex epidemics. nature150 Although rare compared to those diseases that cause the majority of the burden on population health, the nature of such epidemics disrupts health systems, amplifies mistrust among communities and creates high and long-lasting socioeconomic effects, especially in low- and When Nature published its first issue in 18691, a new understanding of middle-income countries. Their increasing frequency demands atten- infectious diseases was taking shape. The work of William Farr2, Ignaz tion.