B COUNCIL REGULATION (EU) No 1387/2013 of 17
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Information to Users
Raman spectroscopic studies of calcium-phosphate, aluminum metaphosphate-sodium fluoride and calcium metaphosphate-calcium fluoride glasses Item Type text; Thesis-Reproduction (electronic) Authors Latifzadeh, Lida, 1956- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 27/09/2021 07:48:35 Link to Item http://hdl.handle.net/10150/278549 INFORMATION TO USERS The most advanced technology has been used to photograph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. -

2020-29037.Pdf
This document is scheduled to be published in the Federal Register on 01/19/2021 and available online at federalregister.gov/d/2020-29037,BILLING and on govinfo.gov CODE 3510-33-P DEPARTMENT OF COMMERCE Bureau of Industry and Security 15 CFR Parts 734, 738, 740, 742, 748, 750, 772, 774 [Docket No. 201221-0350] RIN 0694-AI33 Implementation in the Export Administration Regulations of the United States’ Rescission of Sudan’s Designation as a State Sponsor of Terrorism AGENCY: Bureau of Industry and Security, Commerce. ACTION: Final rule. SUMMARY: In this final rule, the Bureau of Industry and Security (BIS) amends the Export Administration Regulations (EAR) to implement the rescission of Sudan’s designation as a State Sponsor of Terrorism (SSOT). The Secretary of State rescinded this designation effective December 14, 2020 in accordance with established statutory procedures, including the President’s October 26, 2020 submission to Congress of a report justifying the rescission and certifying Sudan had not provided any support for acts of international terrorism during the preceding six month period and that Sudan had provided assurances that it would not support acts of international terrorism in the future. Accordingly, BIS amends the EAR by removing Anti-Terrorism (AT) controls on the country and by removing Sudan from Country Group E:1 (Terrorist supporting countries). These actions render the country eligible for a general 25 percent de minimis level. As a consequence of these actions, as well as the addition of the country to Country Group B, Sudan is also potentially eligible for several new license exceptions under the EAR. -

Development of Novel Peptide Nucleic Acids A
b-Dicarbonyl compounds Compounds having two carbonyl groups separated by an intervening carbon atom are called b-dicarbonyl compounds, and these compounds are highly versatile reagents for organic synthesis. O O O O C C C R C C C OR' b b b-Dicarbonyl system b-Keto ester ** The pKa for such a proton is in the range 9-11, acidic enough to be removed easily by an alkoxide base to form an enolate. O O O H O OR C C C C C.. C + HOR H enolate anion pKa = 9-11 .. .. .. .. .. .. O . O . O O . O O C C C .. C C C R C OR' R C OR' R C OR' H H H Resonance structure of the anion of a b-keto ester Synthesis of b-keto ester (Claisen condensation) O O O NaOC H 2 2 5 CH3COC2H5 CH3CC..HCOC2H5 + C2H5OH + Na (removed by distillation) Sodiumacetoacetic ester HCl O O CH3CCH2COC2H5 Ethyl acetoacetate (acetoacetic ester) (76%) O O O O (1) NaOC2H5 R CH C + H CHC R CH2C CHCOC H + C H OH 2 OC2H5 OC2H5 + 2 5 2 5 (2) H3O R R (R may also be H) b-Keto ester Mechanism Step1 .. O O . .. + . OHC H + C H OH R CHC OC2H5 .. 2 5 RC.. H C OC2H5 2 5 H .. O . RCH C OC2H5 Step 2 .. .. .. .. O . O O O . RCH2C + . RCH2C CH C OC2H5 HC C OC2H5 . C H O . OC2H5 R 2 5 .. R .. O . O .. + . OC2H5 RCH2C CH C OC2H5 .. R Step 3 .. .. O . O H O O . -

Lonza's Chemical Network Diketene and HCN Derivatives, Heterocycles
Lonza’s chemical network Diketene and HCN derivatives, heterocycles and basic chemicals catalog Pharma&Biotech Nutrition Agriculture MaterialsScience PersonalCare Diketene and HCN derivatives, heterocycles and basic chemicals catalog Content About Lonza 3 Introduction 4 Diketene / ketene derivatives 6 Esters 6 Arylides 7 Alkylamides 8 Pyrazolones 9 Dehydroacetic acid 9 Lonzamon monomers 10 Other diketene derivatives 10 HCN derivatives 12 Heterocycles 14 Basic chemicals 16 Others 17 Alphabetical index 18 About Lonza Lonza is the global leader in the production and support of active phar- From 1897 to the present day combining Swiss tradition with global maceutical ingredients both chemically and biotechnologically. Biophar- experience, the company has had an enterprising character, adapting maceuticals are one of the key growth drivers of the pharmaceutical and its offerings and services to the needs of customers and to changing biotechnology industries. technologies. Throughout our history, we have maintained a strong culture of performance, results and dependability that is valued by all Lonza has strong capabilities in large and small molecules, peptides, of our customers. amino acids and niche bioproducts which play an important role in the development of novel medicines and healthcare products. In addition, Lonza’s cracker in Visp is the back bone of a comprehensive fully back- Lonza is a leader in cell-based research, endotoxin detection and cell ward integrated chemical network. Our product portfolio consists of therapy manufacturing. Furthermore, the company is a leading provider HCN- and diketene derivatives as well as basic chemicals which are key of chemical and biotech ingredients to the nutrition, hygiene, preserva- raw materials and intermediates for many sophisticated applications. -

Acetoacetic Ester Synthesis
Programme: B.Sc. B.ed. (Integrated) Course: ORGANIC CHEMISTRY- III Semester: VI Code: CHE-352 Topic: ETHYLACETOACETATEE Date- 07/04/2020 y Only PPe Dr. Angad Kumar Singh ForF Department of Chemistry, Central University of South Bihar, Gaya (Bihar) Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Ethylacetoacetate The Claisen Condensation between esters containing - hdhydrogens, promotdted byabase such as sodium ethoxy ide, toproduce a !- ketoester. One equiv serves as the nucleophilele (enolate)(eno and the other is OnlyO the electrophile which undergoes additiontion andae elimination. The use of stronger bases, e.g. sodium amide or sodiumsodUse hydride instead of sodium ethoxide, often increases the yield.d. al onal Ethylacetoacetate Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Mechanism of the Claisen Condensation The reaction is driven to product by the final deprotonation step. Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Mixed Claisen Condensation Like mixed aldol reactions, mixed Claisen condensations are useful if differences in reactivity exist betweenn they two esters as for example when one of the esters has no -hydrogenydrogOnlyO . Examples of such esters are: e Use ers excess Note: These materials are only for classroom teaching purpose at Central University of South Bihar. -
Classification of Chemicals
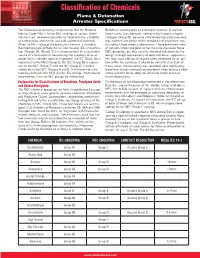
Classification of Chemicals Flame & Detonation Arrester Specifications PROTECTOSEAL ® The Protectoseal Company recommends that the National Butadiene would qualify as a Group D material. In each of Electric Code (NEC) Article 500, rankings of various chemi - these cases, the chemicals were primarly listed in a higher cals be used, whenever possible, to determine the suitability category (Group B), because of relatively high pressure read - of a detonation arrester for use with a particular chemical. ings noted in one phase of the standard test procedure con - When no NEC rating of the particular chemical is available, ducted by Underwriters Laboratories. These pressures were the International Electrotechnical Commission (IEC) classifica - of concern when categorizing the chemicals because these tion (Groups IIA, IIB and IIC) is recommended as a secondary NEC groupings are also used as standard indicators for the source of information for determining the suitability of an ar - design strength requirements of electrical boxes, apparatus, rester for its intended service. In general, the IEC Group IIA is etc. that must withstand the pressures generated by an igni - equivalent to the NEC Group D; the IEC Group IIB is equiva - tion within the container. It should be noted that, in each of lent to the NEC Group C; and the IEC Group IIC includes these cases, the test pressures recorded were significantly chemicals in the NEC Groups A and B. In the event of a dis - lower than those commonly encountered when testing a deto - crepancy between the NEC and the IEC ratings, Protectoseal nation arrester for its ability to withstand stable and over - recommends that the NEC groups be referenced. -

Method for Producing Difluorophosphate
(19) TZZ _T (11) EP 2 826 747 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 21.01.2015 Bulletin 2015/04 C01B 25/455 (2006.01) (21) Application number: 12871541.4 (86) International application number: PCT/JP2012/057408 (22) Date of filing: 14.03.2012 (87) International publication number: WO 2013/136533 (19.09.2013 Gazette 2013/38) (84) Designated Contracting States: • SHOGAMI, Kazuhiko AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Izumiotsu-shi GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Osaka 595-0075 (JP) PL PT RO RS SE SI SK SM TR • SATOH, Tomoya Designated Extension States: Izumiotsu-shi BA ME Osaka 595-0075 (JP) (71) Applicant: Stella Chemifa Corporation (74) Representative: Winter, Brandl, Fürniss, Hübner, Osaka-shi, Osaka 541-0047 (JP) Röss, Kaiser, Polte - Partnerschaft mbB Patent- und Rechtsanwaltskanzlei (72) Inventors: Alois-Steinecker-Strasse 22 • NISHIDA,Tetsuo 85354 Freising (DE) Izumiotsu-shi Osaka 595-0075 (JP) (54) METHOD FOR PRODUCING DIFLUOROPHOSPHATE (57) A process for preparing difluorophosphate com- from the difluorophosphoric acid by solid-liquid separa- prising reacting difluorophosphoric acid with at least one tion, the precipitate being precipitated by crystallization salt, as a raw material, selected from a halide salt, a car- operation in the difluorophosphoric acid, and removing bonate, a phosphate, a hydroxide and an oxide of an the difluorophosphoric acid contained in the precipitate alkali metal, an alkaline earth metal or an onium in the by distillation to obtain difluorophosphate. -

Ethyl Acetoacetate (Acetoacetic Ester) (B) Diethyl Molonote (Molonic Ester) the Structure of (A) and (B) Are As
PRESENTATION OF ENOLATES Dr. Susmita Bajpai Department of Chemistry B.N.D. College, Kanpur ENOLATES The class of compounds which contain a methylene group (–CH2–) directly bonded to the electron withdrawing groups such as –COCH3, –COOC2H5, –CN, are called active methylene compounds. This is so because the –CH2 group in them is acidic and reactive. The two examples are (a) Ethyl acetoacetate (Acetoacetic ester) (b) Diethyl molonote (Molonic ester) The structure of (a) and (b) are as This reaction is known as claisen condensation Ethyl acetoacetate (CH3COOCH2COOC2H5) • It's IUPAC name is ethyl 3-oxobutanoate • Preparation: Ethyl acetoacetate is prepared by heating ethyl acetate with sodium ethoxide in ethanol, followed by acidification. Claisen condensation • It is a condensation reaction in which, two ester molecules condensed to form an alcohol and a b-Keto ester. Therefore ethyl acetoacetate is a b-Keto ester. • Mechanism: The mechanism involves three steps: + • Step-I :- First sodium ethoxide (C2H5O–Na ) breaks into ethoxide ion and sodium ion. This ethoxide ion attacks ethyl acetoacetate to give ethyl alcohol and ester anion. Step -II: Ester anion attacks the carbonyl group of a second molecules of ethyl acetate. • Step-III: Ethoxide ion is eliminated Properties • It is a colourless pleasant smelling liquid • b.p. - 180.4oC • It is sparingly soluble in water but freely so in organic solvent. • It is neutral to litmus. Chemical properties • It is a tautomeric mixture of Keto and enol forms. Therefore it gives the reaction of the various functional groups present in the two forms. Acidity of methylene hydrogen (Formation of salt) • In ethyl acetoacetate methylene group (–CH2–) flank by two carbonyl group. -

Ethyl Acetoacetate
21.6 The Acetoacetic Ester Synthesis Acetoacetic Ester O O C C H3C C OCH2CH3 H H Acetoacetic ester is another name for ethyl acetoacetate. The "acetoacetic ester synthesis" uses acetoacetic ester as a reactant for the preparation of ketones. Deprotonation of Ethyl Acetoacetate O O – C C + CH3CH2O H3C C OCH2CH3 H H Ethyl acetoacetate pKa ~ 11 can be converted readily to its anion with bases such as sodium ethoxide. Deprotonation of Ethyl Acetoacetate O O – C C + CH3CH2O H3C C OCH2CH3 H H Ethyl acetoacetate pKa ~ 11 can be converted readily to its anion K ~ 105 K ~ 10 with bases such as O O sodium ethoxide. C •• C + CH3CH2OH H3C –C OCH2CH3 3 2 H pKa ~ 16 Alkylation of Ethyl Acetoacetate O O The anion of ethyl C •• C acetoacetate can be H C C OCH CH 3 – C OCH2CH3 alkylated using an H alkyl halide (SN2: primary and R X secondary alkyl halides work best; tertiary alkyl halides undergo elimination). Alkylation of Ethyl Acetoacetate O O The anion of ethyl C •• C acetoacetate can be H C C OCH CH 3 – C OCH2CH3 alkylated using an H alkyl halide (SN2: primary and R X secondary alkyl O O halides work best; tertiary alkyl halides C C undergo elimination). H3C C OCH2CH3 H R Conversion to Ketone O O Saponification and C C acidification convert H3C C OH the alkylated H R derivative to the – 1. HO , H2O corresponding b-keto 2. H+ acid. O O The b-keto acid then undergoes C C decarboxylation to H C 3 C OCH2CH3 form a ketone. -

Determination of Solvent Effects on Ketoðenol Equilibria of 1,3-Dicarbonyl Compounds Using NMR: Revisiting a Classic Physical C
In the Laboratory Determination of Solvent Effects on Keto–Enol Equilibria W of 1,3-Dicarbonyl Compounds Using NMR Revisiting a Classic Physical Chemistry Experiment Gilbert Cook* and Paul M. Feltman Department of Chemistry, Valparaiso University, Valparaiso, IN 46383; *[email protected] “The influence of solvents on chemical equilibria was discovered in 1896, simultaneously with the discovery of keto–enol tautomerism in 1,3-dicarbonyl compounds” (1). The solvents were divided into two groups according to their ability to isomerize compounds. The study of the keto–enol tautomerism of β-diketones and β-ketoesters in a variety of solvents using proton NMR has been utilized as a physical Figure 1. The β-dicarbonyl compounds studied in the experiment. chemistry experiment for many years (2, 3). The first reported use of NMR keto–enol equilibria determination was by Reeves (4). This technique has been described in detail in an experiment by Garland, Nibler, and Shoemaker (2). panded (i) to give an in-depth analysis of factors influencing The most commonly used β-diketone for these experi- solvent effects in tautomeric equilibria and (ii) to illustrate ments is acetylacetone (Scheme I). Use of proton NMR is a the use of molecular modeling in determining the origin of viable method for measuring this equilibrium because the a molecule’s polarity. The experiment’s original benefits of tautomeric keto–enol equilibrium is slow on the NMR time using proton NMR as a noninvasive method of evaluating scale, but enol (2a)–enol (2b) tautomerism is fast on this scale equilibrium are maintained. (5). It has been observed that acyclic β-diketones and β- Experimental Procedure ketoesters follow Meyer’s rule of a shift in the tautomeric equi- librium toward the keto tautomer with increasing solvent Observations of the solvent effects for three other 1,3- polarity (6). -

Atoa Wa Ni Mha La Maison
ATOAUS009975847B2 WA NI MHA LA MAISON (12 ) United States Patent ( 10 ) Patent No. : US 9 ,975 , 847 B2 Gambogi et al. (45 ) Date of Patent: May 22 , 2018 ( 54 ) AMINO ACID DERIVATIVES AND THEIR 2006 /0013778 A1 1 / 2006 Hodosh 2008/ 0317839 Al * 12/ 2008 Quay .. .. .. .. .. A61K 9 / 1272 USES 424 / 450 ( 71) Applicant: Johnson & Johnson Consumer Inc ., 2010 /0330136 Al 12 /2010 Rocabayera Bonvila Skillman , NJ (US ) FOREIGN PATENT DOCUMENTS (72 ) Inventors : Robert J . Gambogi, Hillsborough , NJ JP 5982310 5 / 1984 (US ) ; Anthony R . Geonnotti, III , WO WO2000 /011022 2 / 2000 Princeton , NJ (US ) ; Michael C . Giano , WO WO2003 /013454 2 / 2003 Southampton , NJ ( US ) ; Latrisha WO WO 2005 /000261 AL 1 /2005 Petersen , Highland Park , NJ (US ) WO WO2008 / 137758 A2 11/ 2008 (73 ) Assignee : Johnson & Johnson Consumer Inc . , OTHER PUBLICATIONS Skillman , NJ (US ) Kazuhiko et al . JPS 5982310 ( A ) ( 1984 ) English Abstracts . * Pinazo , A . ; New cationic vesicles prepared with double chain ( * ) Notice : Subject to any disclaimer , the term of this surfactants fromarginine: Role of the hydrophobic group on the patent is extended or adjusted under 35 antimicrobial activityand cytotoxicity ; Colloids and Surfaces B : U . S . C . 154 (b ) by 15 days. Biointerfaces 141 ( 2016 ) 19 - 27 . International Search Report dated Jan . 21, 2016 ; — Int' l Appln . No. ( 21) Appl . No. : 14 /938 , 334 PCT/ US2015 / 060166 filed Nov . 11, 2015 . Morrison and Boyd , Organic Chemistry Fourth Edition , section ( 22 ) Filed : Nov . 11 , 2015 20 . 3 , p . 814 . Yang Xu and R . F . Pratt , “ -Lactam -Recognizing Enzymes Exhibit (65 ) Prior Publication Data Different Structural Specificity in Acyclic Amide and Ester Sub strates : A Starting point in B -Lactamase Evolution ? ” Bioorganic & US 2016 /0145203 A1 May 26 , 2016 Medicinal Chemistry Letters, vol . -

Ethyl Acetoacetate
EUROPEAN COMMISSION JOINT RESEARCH CENTRE Institute for Health and Consumer Protection European Chemicals Bureau I-21020 Ispra (VA) Italy ETHYL ACETOACETATE CAS No: 141-97-9 EINECS No: 205-516-1 Summary Risk Assessment Report 2002 Special Publication I.02.75 ETHYL ACETOACETATE CAS No: 141-97-9 EINECS No: 205-516-1 SUMMARY RISK ASSESSMENT REPORT 2002 Germany The Rapporteur for Ethyl acetoacetate is the Federal Institute for Occupational Safety and Health. Contact point: Bundesanstalt für Arbeitsschutz und Arbeitsmedizin Anmeldestelle Chemikaliengesetz (BAuA) (Federal Institute for Occupational Safety and Health Notification Unit) Friedrich-Henkel-Weg 1-25 44149 Dortmund (Germany) fax: +49 (231) 9071-679 e-mail: [email protected] Date of Last Literature Search : 1996 Review of report by MS Technical Experts finalised: March 2001 Final report: 2002 © European Communities, 2002 PREFACE This report provides a summary, with conclusions, of the risk assessment report of the substance ethyl acetoacetate that has been prepared by Germany in the context of Council Regulation (EEC) No. 793/93 on the evaluation and control of existing substances. For detailed information on the risk assessment principles and procedures followed, the underlying data and the literature references the reader is referred to the original risk assessment report that can be obtained from the European Chemicals Bureau1. The present summary report should preferably not be used for citation purposes. 1 European Chemicals Bureau – Existing Chemicals – http://ecb.jrc.it III