Atoa Wa Ni Mha La Maison
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Information to Users
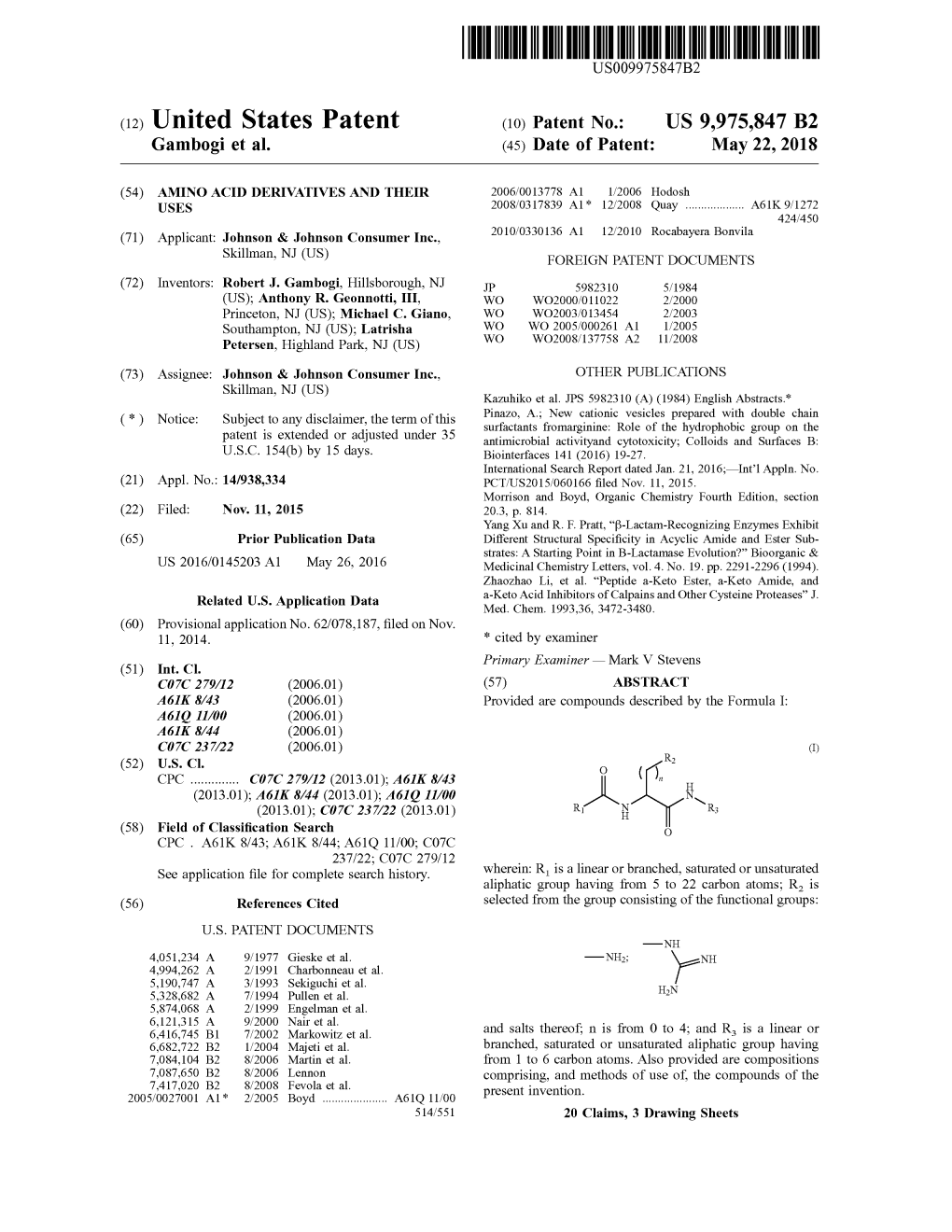
Raman spectroscopic studies of calcium-phosphate, aluminum metaphosphate-sodium fluoride and calcium metaphosphate-calcium fluoride glasses Item Type text; Thesis-Reproduction (electronic) Authors Latifzadeh, Lida, 1956- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 27/09/2021 07:48:35 Link to Item http://hdl.handle.net/10150/278549 INFORMATION TO USERS The most advanced technology has been used to photograph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. -

2020-29037.Pdf
This document is scheduled to be published in the Federal Register on 01/19/2021 and available online at federalregister.gov/d/2020-29037,BILLING and on govinfo.gov CODE 3510-33-P DEPARTMENT OF COMMERCE Bureau of Industry and Security 15 CFR Parts 734, 738, 740, 742, 748, 750, 772, 774 [Docket No. 201221-0350] RIN 0694-AI33 Implementation in the Export Administration Regulations of the United States’ Rescission of Sudan’s Designation as a State Sponsor of Terrorism AGENCY: Bureau of Industry and Security, Commerce. ACTION: Final rule. SUMMARY: In this final rule, the Bureau of Industry and Security (BIS) amends the Export Administration Regulations (EAR) to implement the rescission of Sudan’s designation as a State Sponsor of Terrorism (SSOT). The Secretary of State rescinded this designation effective December 14, 2020 in accordance with established statutory procedures, including the President’s October 26, 2020 submission to Congress of a report justifying the rescission and certifying Sudan had not provided any support for acts of international terrorism during the preceding six month period and that Sudan had provided assurances that it would not support acts of international terrorism in the future. Accordingly, BIS amends the EAR by removing Anti-Terrorism (AT) controls on the country and by removing Sudan from Country Group E:1 (Terrorist supporting countries). These actions render the country eligible for a general 25 percent de minimis level. As a consequence of these actions, as well as the addition of the country to Country Group B, Sudan is also potentially eligible for several new license exceptions under the EAR. -

Method for Producing Difluorophosphate
(19) TZZ _T (11) EP 2 826 747 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 21.01.2015 Bulletin 2015/04 C01B 25/455 (2006.01) (21) Application number: 12871541.4 (86) International application number: PCT/JP2012/057408 (22) Date of filing: 14.03.2012 (87) International publication number: WO 2013/136533 (19.09.2013 Gazette 2013/38) (84) Designated Contracting States: • SHOGAMI, Kazuhiko AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Izumiotsu-shi GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Osaka 595-0075 (JP) PL PT RO RS SE SI SK SM TR • SATOH, Tomoya Designated Extension States: Izumiotsu-shi BA ME Osaka 595-0075 (JP) (71) Applicant: Stella Chemifa Corporation (74) Representative: Winter, Brandl, Fürniss, Hübner, Osaka-shi, Osaka 541-0047 (JP) Röss, Kaiser, Polte - Partnerschaft mbB Patent- und Rechtsanwaltskanzlei (72) Inventors: Alois-Steinecker-Strasse 22 • NISHIDA,Tetsuo 85354 Freising (DE) Izumiotsu-shi Osaka 595-0075 (JP) (54) METHOD FOR PRODUCING DIFLUOROPHOSPHATE (57) A process for preparing difluorophosphate com- from the difluorophosphoric acid by solid-liquid separa- prising reacting difluorophosphoric acid with at least one tion, the precipitate being precipitated by crystallization salt, as a raw material, selected from a halide salt, a car- operation in the difluorophosphoric acid, and removing bonate, a phosphate, a hydroxide and an oxide of an the difluorophosphoric acid contained in the precipitate alkali metal, an alkaline earth metal or an onium in the by distillation to obtain difluorophosphate. -

Alfa Laval Black and Grey List, Rev 14.Pdf 2021-02-17 1678 Kb
Alfa Laval Group Black and Grey List M-0710-075E (Revision 14) Black and Grey list – Chemical substances which are subject to restrictions First edition date. 2007-10-29 Revision date 2021-02-10 1. Introduction The Alfa Laval Black and Grey List is divided into three different categories: Banned, Restricted and Substances of Concern. It provides information about restrictions on the use of Chemical substances in Alfa Laval Group’s production processes, materials and parts of our products as well as packaging. Unless stated otherwise, the restrictions on a substance in this list affect the use of the substance in pure form, mixtures and purchased articles. - Banned substances are substances which are prohibited1. - Restricted substances are prohibited in certain applications relevant to the Alfa Laval group. A restricted substance may be used if the application is unmistakably outside the scope of the legislation in question. - Substances of Concern are substances of which the use shall be monitored. This includes substances currently being evaluated for regulations applicable to the Banned or Restricted categories, or substances with legal demands for monitoring. Product owners shall be aware of the risks associated with the continued use of a Substance of Concern. 2. Legislation in the Black and Grey List Alfa Laval Group’s Black and Grey list is based on EU legislations and global agreements. The black and grey list does not correspond to national laws. For more information about chemical regulation please visit: • REACH Candidate list, Substances of Very High Concern (SVHC) • REACH Authorisation list, SVHCs subject to authorization • Protocol on persistent organic pollutants (POPs) o Aarhus protocol o Stockholm convention • Euratom • IMO adopted 2015 GUIDELINES FOR THE DEVELOPMENT OF THE INVENTORY OF HAZARDOUS MATERIALS” (MEPC 269 (68)) • The Hong Kong Convention • Conflict minerals: Dodd-Frank Act 1 Prohibited to use, or put on the market, regardless of application. -

Federal Register/Vol. 85, No. 117/Wednesday, June 17, 2020
Federal Register / Vol. 85, No. 117 / Wednesday, June 17, 2020 / Rules and Regulations 36483 other person (except an airman serving 1C350 is amended by adding twenty- program. The AG periodically reviews as an airman) not operating an aircraft four precursor chemicals, as well as items on its control list to enhance the for the transportation of passengers or mixtures in which at least one of these effectiveness of participating property for compensation. For chemicals constitutes 30 percent or governments’ national controls and to violations that occurred after November more of the weight of the mixture, to achieve greater harmonization among 2, 2015, $13,910 per violation, up to a ECCN 1C350.d. ECCN 1C351 is these controls. total of $556,419 per civil penalty amended to add Middle East respiratory Amendments to the CCL Based on the action, in the case of any other person syndrome-related coronavirus (MERS- February 2020 AG Intersessional (except an airman serving as an airman) related coronavirus). ECCN 2B352 is Recommendations not operating an aircraft for the amended by adding a Technical Note to transportation of passengers or property indicate that cultivation chamber ECCN 1C350 (Chemical Weapons for compensation. holding devices controlled in Precursors) (3) For violations that occurred on or 2B352.b.2.b include single-use This final rule amends Export Control before November 2, 2015, $25,000 per cultivation chambers with rigid walls. Classification Number (ECCN) 1C350 on violation, up to a total of $400,000 per The items addressed by this final rule the Commerce Control List (CCL) civil penalty action, in the case of a were not previously listed on the CCL (Supplement No. -

Export Controlled Chemicals, Including Mixtures and Compounds
Export Controlled Chemicals, including mixtures and compounds **ALL High Explosives and their Precursors Are Export Controlled** Note that mixtures in which at least one of the chemicals listed below constitutes 30 percent or more of the weight of the mixture are also controlled. - 1,1-Diethylhydrazine nitrate (DEHN)/ 1,2-Diethylhydrazine nitrate (DEHN) (CAS 363453- 17-2) - 1,1-Dimethylhydrazinium azide (CAS 227955-52-4)/ - 1,2-Dimethylhydrazinium azide (CAS 299177-50-7) - 2 Nitrodiphenylamine (2-NDPA) - 2-Chloroethanol (CAS 107-07-3) - 2-hydroxyethylhydrazine nitrate (HEHN) - 3-Hydroxyl-1-methylpiperidine (CAS 3554-74-3) - 3-Quinuclidinol (CAS 1619-34-7) - 3-Quinuclidone (CAS 3731-38-2) - 3,6-dihydrazino tetrazine nitrate (DHTN), also referred to as 1,4-dihydrazine nitrate. - Allylhydrazine (CAS 7422-78-8) - Ammonium hydrogen fluoride (CAS 1341-49-7) - Ammonium nitrate (including fertilizers) containing more than 15% by weight ammonium nitrate - Arsenic trichloride (CAS 7784-34-1) - Benzilic acid (CAS 76-93-7) - Carboxy-terminated polybutadiene (including carboxyl-terminated polybutadiene) (CTPB) - Chemicals containing a phosphorus atom to which is bonded one methyl, ethyl, or propyl (normal or iso) group but not further carbon atoms. - Chlorine trifluoride (ClF3) - Chloropicrin: Trichloronitromethane (CAS 76-06-2) - Cyanogen chloride (CAS 506-77-4) - Di-isopropylamine (CAS 108-18-9) - Diethyl chlorophosphite (CAS 589–57–1) - Diethyl ethylphosphonate (CAS 78-38-6) - Diethyl methylphosphonate (CAS 683-08-9) - Diethyl methylphosphonite -

Lithium Difluorophosphate, Electrolytic Solution
(19) & (11) EP 2 061 115 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 20.05.2009 Bulletin 2009/21 H01M 10/40 (2006.01) C01B 25/455 (2006.01) (21) Application number: 07792892.7 (86) International application number: PCT/JP2007/066306 (22) Date of filing: 22.08.2007 (87) International publication number: WO 2008/023744 (28.02.2008 Gazette 2008/09) (84) Designated Contracting States: (71) Applicant: Mitsubishi Chemical Corporation AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Minato-ku HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE Tokyo 108-0014 (JP) SI SK TR Designated Extension States: (72) Inventor: TAKEHARA, Masahiro AL BA HR MK RS Ibaraki 300-0332 (JP) (30) Priority: 22.08.2006 JP 2006225409 (74) Representative: HOFFMANN EITLE 02.11.2006 JP 2006299360 Patent- und Rechtsanwälte Arabellastrasse 4 81925 München (DE) (54) LITHIUM DIFLUOROPHOSPHATE, ELECTROLYTIC SOLUTION CONTAINING LITHIUM DIFLUOROPHOSPHATE, PROCESS FOR PRODUCING LITHIUM DIFLUOROPHOSPHATE, PROCESS FOR PRODUCING NONAQUEOUS ELECTROLYTIC SOLUTION, NONAQUEOUS ELECTROLYTIC SOLUTION, AND NONAQUEOUS-ELECTROLYTIC-SOLUTION SECONDARY CELL EMPLOYING THE SAME (57) A difluorophosphate salt, which is expensive and not readily available, can be produced with a high purity readily and efficiently from inexpensive and readily available materials. A nonaqueous electrolyte secondary battery that exhibits low-temperature discharge and heavy-current discharge characteristics and high-temperature preservation and cycle -

UK Strategic Export Control Lists
UK Strategic Export Control Lists The consolidated list of strategic military and dual-use items that require export authorisation from Great Britain and Northern Ireland January 2021 UK STRATEGIC EXPORT CONTROL LISTS Contents • Introduction • UK Military List [Schedule 2 to the Export Control Order 2008] • UK Dual-Use List [Schedule 3 to the Export Control Order 2008] • Non-military Firearms List [Annex I to Regulation1 No. 258/2012] • Human Rights List [Annexes II, III & IV of Regulation1 No. 125/2019] • UK Security and Human Rights List [Articles 4A and 42S to the Export Control Order 2008] • UK Radioactive Source List [Schedule to the Export of Radioactive Sources (Control) Order 2006] • Dual-Use List [Annex I to Regulation (EC)1 No. 428/2009]: o Category 0 Nuclear materials, facilities and equipment o Category 1 Special materials and related equipment o Category 2 Materials processing o Category 3 Electronics o Category 4 Computers o Category 5 Telecommunications and "information security" o Category 6 Sensors and lasers o Category 7 Navigation and avionics o Category 8 Marine o Category 9 Aerospace and Propulsion • Dual-Use List [Annex IV to Regulation (EC)1 No. 428/2009] The control text reproduced in this guide is for information purposes only and has no force in law. Please note that where legal advice is required, exporters should make their own arrangements. 1 Retained Regulation applies in Great Britain and the EU Regulation applies in Northern Ireland Strategic Export Control Lists: Contents and Introduction - Page 1 INTR_U26.docx 31 December 2020 Introduction These lists summarise the text from the appropriate strategic export control legislation (excluding any Sanctions Orders) that was in force at 11pm on the 31 December 2020 for UK exports. -

Category 1—Page 1
Commerce Control List Supplement No. 1 to Part 774 Category 1—page 1 CATEGORY 1 - SPECIAL MATERIALS AND to the ITAR” (see 22 CFR parts 120 through RELATED EQUIPMENT, CHEMICALS, 130, including USML Category XXI). (2) “MICROORGANISMS,” AND “TOXINS” See also 1C009. Related Definitions: N/A Note: The Food and Drug Administration Items: (FDA) and the Drug Enforcement Administration (DEA) may control exports of items subject to the a. Seals, gaskets, sealants or fuel bladders, EAR and on the Commerce Control List. BIS “specially designed” for “aircraft” or aerospace provides cross references to these other agency use, made from more than 50% by weight of any controls for convenience only. Therefore, please of the materials controlled by 1C009.b or consult relevant FDA and DEA regulations for 1C009.c; guidance related to the item you wish to export and do not rely solely on the EAR for information b. [Reserved] about other agency export control requirements. See Supplement No. 3 to part 730 (Other U.S. Government Departments and Agencies with 1A002 “Composite” structures or laminates, Export Control Responsibilities) for as follows (see List of Items Controlled). more information. License Requirements A. “END ITEMS,” “EQUIPMENT,” Reason for Control: NS, NP, AT “ACCESSORIES,” “ATTACHMENTS,” “PARTS,” “COMPONENTS,” AND Control(s) Country Chart “SYSTEMS” (See Supp. No. 1 to part 738) 1A001 “Parts” and “components” made from NS applies to entire entry NS Column 2 fluorinated compounds, as follows (see List of NP applies to 1A002.b.1 in NP Column 1 Items Controlled). the form of tubes with an inside diameter between 75 License Requirements mm and 400 mm AT applies to entire entry AT Column 1 Reason for Control: NS, AT Reporting Requirements Country Chart Control(s) (See Supp. -

B COUNCIL REGULATION (EU) No 1387/2013 of 17
02013R1387 — EN — 01.01.2019 — 010.001 — 1 This text is meant purely as a documentation tool and has no legal effect. The Union's institutions do not assume any liability for its contents. The authentic versions of the relevant acts, including their preambles, are those published in the Official Journal of the European Union and available in EUR-Lex. Those official texts are directly accessible through the links embedded in this document ►B COUNCIL REGULATION (EU) No 1387/2013 of 17 December 2013 suspending the autonomous Common Customs Tariff duties on certain agricultural and industrial products and repealing Regulation (EU) No 1344/2011 (OJ L 354, 28.12.2013, p. 201) Amended by: Official Journal No page date ►M1 Council Regulation (EU) No 722/2014 of 24 June 2014 L 192 9 1.7.2014 ►M2 Council Regulation (EU) No 1341/2014 of 15 December 2014 L 363 10 18.12.2014 ►M3 Council Regulation (EU) 2015/982 of 23 June 2015 L 159 5 25.6.2015 ►M4 Council Regulation (EU) 2015/2449 of 14 December 2015 L 345 11 30.12.2015 ►M5 Council Regulation (EU) 2016/1051 of 24 June 2016 L 173 5 30.6.2016 ►M6 Council Regulation (EU) 2016/2390 of 19 December 2016 L 360 14 30.12.2016 ►M7 Council Regulation (EU) 2017/1134 of 20 June 2017 L 164 6 27.6.2017 ►M8 Council Regulation (EU) 2017/2467 of 21 December 2017 L 351 7 30.12.2017 ►M9 Council Regulation (EU) 2018/914 of 25 June 2018 L 162 8 27.6.2018 ►M10 Council Regulation (EU) 2018/2069 of 20 December 2018 L 331 4 28.12.2018 Corrected by: ►C1 Corrigendum, OJ L 293, 9.10.2014, p. -

Commerce Control List - Index Supplement No
Commerce Control List - Index Supplement No. 1 to part 774 - Index 1 ALPHABETICAL INDEX TO THE COMMERCE CONTROL LIST This index is not an exhaustive list of controlled items. Supplement No. 4 to Part 774 – Commerce Control List Order of Review identifies the steps to follow when reviewing the Commerce Control List. Description ECCN Citation Ablative liners, thrust or combustion chambers ............................................. 9A106.a, 9A619.c, .e Abrin ................................................................................................................................. 1C351.d.1 Absolute reflectance measurement equipment .................................................................... 6B004.a Absorbers of electromagnetic waves ...................................................................................... 1C001 Absorbers, hair type ...............................................................................................1C001.a Note 1.a Absorbers, non-planar & planar ............................................................ 1C001.a Note 1, b, c, d or e Absorbers, planar ...................................................................................... 1C001.a, Note 1.c, d or e Absorption columns ............................................................................................................. 2B350.e Accelerators (electro-magnetic radiation) ............................................................................ 3A101.b Accelerometer axis align stations .......................................................................... -

Difluorophosphate Production Method
(19) TZZ __T (11) EP 2 899 157 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 29.07.2015 Bulletin 2015/31 C01B 25/455 (2006.01) (21) Application number: 14807360.4 (86) International application number: PCT/JP2014/065100 (22) Date of filing: 06.06.2014 (87) International publication number: WO 2014/196632 (11.12.2014 Gazette 2014/50) (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • KON Sojiro GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Izumiotsu-shi PL PT RO RS SE SI SK SM TR Osaka 595-0075 (JP) Designated Extension States: • NISHIDA Tetsuo BA ME Izumiotsu-shi Osaka 595-0075 (JP) (30) Priority: 07.06.2013 JP 2013121330 (74) Representative: Sajda, Wolf E. (71) Applicant: Stella Chemifa Corporation Meissner, Bolte & Partner GbR Osaka 541-0047 (JP) Postfach 86 06 24 81633 München (DE) (54) DIFLUOROPHOSPHATE PRODUCTION METHOD (57) Provided is a method for producing a difluoro- difluorophosphate in the crude difluorophosphoric acid; phosphate, which can simply and easily produce a high- and heating and drying the crude difluorophosphoric acid purity difluorophosphate in an industrially advantageous containing the difluorophosphate to distill away the crude manner. The method includes steps of: reacting an aque- difluorophosphoricacid, or precipitating the difluorophos- ous hydrofluoric acid solution with an oxyhalide of phos- phate in the crude difluorophosphoric acid by crystalliza- phorous (except phosphoric trifluoride) to produce a tion, subsequently separating the difluorophosphate by crude difluorophosphoric acid; reacting the crude difluor- solid-liquid separation, and further distilling away the ophosphoric acid with a halide of an alkali metal, an al- crude difluorophosphoric acid contained in the difluoro- kaline earth metal, aluminum or an onium to produce a phosphate after solid-liquid separation.