Uranium-232 Beryllide Neutron Source
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
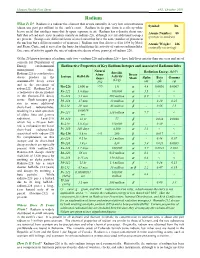
Radium What Is It? Radium Is a Radioactive Element That Occurs Naturally in Very Low Concentrations Symbol: Ra (About One Part Per Trillion) in the Earth’S Crust
Human Health Fact Sheet ANL, October 2001 Radium What Is It? Radium is a radioactive element that occurs naturally in very low concentrations Symbol: Ra (about one part per trillion) in the earth’s crust. Radium in its pure form is a silvery-white heavy metal that oxidizes immediately upon exposure to air. Radium has a density about one- Atomic Number: 88 half that of lead and exists in nature mainly as radium-226, although several additional isotopes (protons in nucleus) are present. (Isotopes are different forms of an element that have the same number of protons in the nucleus but a different number of neutrons.) Radium was first discovered in 1898 by Marie Atomic Weight: 226 and Pierre Curie, and it served as the basis for identifying the activity of various radionuclides. (naturally occurring) One curie of activity equals the rate of radioactive decay of one gram (g) of radium-226. Of the 25 known isotopes of radium, only two – radium-226 and radium-228 – have half-lives greater than one year and are of concern for Department of Energy environmental Radioactive Properties of Key Radium Isotopes and Associated Radionuclides management sites. Natural Specific Radiation Energy (MeV) Radium-226 is a radioactive Abun- Decay Isotope Half-Life Activity decay product in the dance Mode Alpha Beta Gamma (Ci/g) uranium-238 decay series (%) (α) (β) (γ) and is the precursor of Ra-226 1,600 yr >99 1.0 α 4.8 0.0036 0.0067 radon-222. Radium-228 is a radioactive decay product Rn-222 3.8 days 160,000 α 5.5 < < in the thorium-232 decay Po-218 3.1 min 290 million α 6.0 < < series. -

MASTER 9700 South Cass Avenue Argonne, Illinois 60439 USA
ANL/KDH—SO DE84 001440 ANL/NDM-80 NEUTRON TOTAL CROSS SECTION MEASUREMENTS IN THE ENERGY REGION FROM 47 key to 20 MeV* by W. P. Poenitz and J. F. Whalon Applied Physics Division May, 1983 *This work supported by the U.S. Department of Energy Argonne National Laboratory MASTER 9700 South Cass Avenue Argonne, Illinois 60439 USA fflSTRtBtfUOU OF miS DOCUMENT IS UNLIMITED NUCLEAR DATA AMD MEASUREMENTS SERIES The Nuclear Data and Measurements Series presents results of studies in the field of microscopic nuclear data. The primary objective is the dissemination of information in the comprehensive form required for nuclear technology applications. This Series is devoted to: a) measured microscopic nuclear parameters, b) experimental techniques and facilities employed in measurements, c) the analysis, correlation and interpretation of nuclear data, and d) the evaluation of nuclear data. Contributions to this Series are reviewed to assure technical competence and, unless otherwise stated, the contents can be formally referenced. This Series does not supplant formal journal publication but it does provide the more extensive informa- tion required for technological applications (e.g., tabulated numerical data) in a timely manner. DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or retpoosi- bility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Refer- ence herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recom- mendation, or favoring by the United States Government or any agency thereof. -

The Use of Cosmic-Rays in Detecting Illicit Nuclear Materials
The use of Cosmic-Rays in Detecting Illicit Nuclear Materials Timothy Benjamin Blackwell Department of Physics and Astronomy University of Sheffield This dissertation is submitted for the degree of Doctor of Philosophy 05/05/2015 Declaration I hereby declare that except where specific reference is made to the work of others, the contents of this dissertation are original and have not been submitted in whole or in part for consideration for any other degree or qualification in this, or any other Univer- sity. This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration, except where specifically indicated in the text. Timothy Benjamin Blackwell 05/05/2015 Acknowledgements This thesis could not have been completed without the tremendous support of many people. Firstly I would like to express special appreciation and thanks to my academic supervisor, Dr Vitaly A. Kudryavtsev for his expertise, understanding and encourage- ment. I have enjoyed our many discussions concerning my research topic throughout the PhD journey. I would also like to thank my viva examiners, Professor Lee Thomp- son and Dr Chris Steer, for the time you have taken out of your schedules, so that I may take this next step in my career. Thanks must also be given to Professor Francis Liven, Professor Neil Hyatt and the rest of the Nuclear FiRST DTC team, for the initial oppor- tunity to pursue postgraduate research. Appreciation is also given to the University of Sheffield, the HEP group and EPRSC for providing me with the facilities and funding, during this work. -

Uses of Isotopic Neutron Sources in Elemental Analysis Applications
EG0600081 3rd Conference on Nuclear & Particle Physics (NUPPAC 01) 20 - 24 Oct., 2001 Cairo, Egypt USES OF ISOTOPIC NEUTRON SOURCES IN ELEMENTAL ANALYSIS APPLICATIONS A. M. Hassan Department of Reactor Physics Reactors Division, Nuclear Research Centre, Atomic Energy Authority. Cairo-Egypt. ABSTRACT The extensive development and applications on the uses of isotopic neutron in the field of elemental analysis of complex samples are largely occurred within the past 30 years. Such sources are used extensively to measure instantaneously, simultaneously and nondestruclively, the major, minor and trace elements in different materials. The low residual activity, bulk sample analysis and high accuracy for short lived elements are improved. Also, the portable isotopic neutron sources, offer a wide range of industrial and field applications. In this talk, a review on the theoretical basis and design considerations of different facilities using several isotopic neutron sources for elemental analysis of different materials is given. INTRODUCTION In principle there are two ways to use neutrons for elemental and isotopic abundance analysis in samples. One is the neutron activation analysis which we call it the "off-line" where the neutron - induced radioactivity is observed after the end of irradiation. The other one we call it the "on-line" where the capture gamma-rays is observed during the neutron bombardment. Actually, the sequence of events occurring during the most common type of nuclear reaction used in this analysis namely the neutron capture or (n, gamma) reaction, is well known for the people working in this field. The neutron interacts with the target nucleus via a non-elastic collision, a compound nucleus forms in an excited state. -

PGNAA Neutron Source Moderation Setup Optimization
Submitted to ‘Chinese Physics C PGNAA neutron source moderation setup optimization Zhang Jinzhao1(张金钊)Tuo Xianguo1(庹先国) (1.Chengdu University of Technology Applied Nuclear Techniques in Geoscience Key Laboratory of Sichuan Province,Chengdu 610059,China) Abstract: Monte Carlo simulations were carried out to design a prompt γ-ray neutron activation analysis (PGNAA) thermal neutron output setup using MCNP5 computer code. In these simulations the moderator materials, reflective materials and structure of the PGNAA 252Cf neutrons of thermal neutron output setup were optimized. Results of the calcuations revealed that the thin layer paraffin and the thick layer of heavy water moderated effect is best for 252Cf neutrons spectrum. The new design compared with the conventional neutron source design, the thermal neutron flux and rate were increased by 3.02 times and 3.27 times. Results indicate that the use of this design should increase the neutron flux of prompt gamma-ray neutron activation analysis significantly. Key word: PGNAA; neutron source; thermal neutron; moderation; reflection 1. Introduction study, Monte Carlo calculation was carried out for the Prompt gamma ray neutron activation analysis design of a 252Cf neutron source moderation setup for the (PGNAA) is a rapid, nondestructive, powerful analysis cement samples[7]. The model of Monte Carlo multielemental analysis technique, large samples of some simulation was verified by experiment[8, 9].We improve minor, trace light elements and is used in industrial the thermal neutron source yield rate of 252Cf neutron by control[1-5]. In a PGNAA analysis, the sample nuclear the PGNAA neutron source structure to the design. The composition is determined from prompt gamma rays calculation results for the new design were compared which produced through neutron inelastic scattering and with the previous, example: themal neutron flux rate, fast thermal neutron capture. -

Two-Proton Radioactivity 2
Two-proton radioactivity Bertram Blank ‡ and Marek P loszajczak † ‡ Centre d’Etudes Nucl´eaires de Bordeaux-Gradignan - Universit´eBordeaux I - CNRS/IN2P3, Chemin du Solarium, B.P. 120, 33175 Gradignan Cedex, France † Grand Acc´el´erateur National d’Ions Lourds (GANIL), CEA/DSM-CNRS/IN2P3, BP 55027, 14076 Caen Cedex 05, France Abstract. In the first part of this review, experimental results which lead to the discovery of two-proton radioactivity are examined. Beyond two-proton emission from nuclear ground states, we also discuss experimental studies of two-proton emission from excited states populated either by nuclear β decay or by inelastic reactions. In the second part, we review the modern theory of two-proton radioactivity. An outlook to future experimental studies and theoretical developments will conclude this review. PACS numbers: 23.50.+z, 21.10.Tg, 21.60.-n, 24.10.-i Submitted to: Rep. Prog. Phys. Version: 17 December 2013 arXiv:0709.3797v2 [nucl-ex] 23 Apr 2008 Two-proton radioactivity 2 1. Introduction Atomic nuclei are made of two distinct particles, the protons and the neutrons. These nucleons constitute more than 99.95% of the mass of an atom. In order to form a stable atomic nucleus, a subtle equilibrium between the number of protons and neutrons has to be respected. This condition is fulfilled for 259 different combinations of protons and neutrons. These nuclei can be found on Earth. In addition, 26 nuclei form a quasi stable configuration, i.e. they decay with a half-life comparable or longer than the age of the Earth and are therefore still present on Earth. -
![Arxiv:1506.05417V2 [Physics.Ins-Det] 28 Jul 2016](https://docslib.b-cdn.net/cover/1390/arxiv-1506-05417v2-physics-ins-det-28-jul-2016-561390.webp)
Arxiv:1506.05417V2 [Physics.Ins-Det] 28 Jul 2016
http://dx.doi.org/10.1016/j.apradiso.2016.06.032 A precise method to determine the activity of a weak neutron source using a germanium detector M. J. M. Dukea, A. L. Hallinb, C. B. Kraussb, P. Mekarskib,∗, L. Sibleyb aSLOWPOKE Nuclear Reactor Facility, University of Alberta, Edmonton, AB T6G 2G7, Canada bDepartment of Physics, University of Alberta, Edmonton, AB T6G 2E1, Canada Abstract A standard high purity germanium (HPGe) detector was used to determine the previously unknown neutron activity of a weak americium-beryllium (AmBe) neutron source. γ rays were created through 27Al(n,n0), 27Al(n,γ) and 1H(n,γ) reactions induced by the neutrons on aluminum and acrylic disks, respectively. These γ rays were measured using the HPGe detector. Given the unorthodox experimental arrangement, a Monte Carlo simulation was developed to model the efficiency of the detector system to determine the neutron activity from the measured γ rays. The activity of our neutron source was determined to be 307.4 ± 5.0 n/s and is consistent for the different neutron-induced γ rays. Keywords: neutron activation, germanium detector, simulation, spectroscopy, activity determination 1. Introduction As neutrons are difficult to detect, determining the absolute activity of a neutron source is challenging. This difficulty increases as the activity of the source decreases. Sophisticated techniques exist for neutron activity measure- ments, including the manganese bath technique[1], proton recoil techniques[2] and the use of 3He proportional counters[3]; nevertheless, the development of a method utilizing commonly available high purity germanium (HPGe) detectors would be advantageous. HPGe's are an industry standard for measuring γ ray energies to high preci- sion. -

"Indoor Radon and Radon Decay Product Measurement Device Protocols"
"Indoor Radon and Radon Decay Product Measurement Device Protocols" U.S. Environmental Protection Agency Office of Air and Radiation (6604J) EPA 402-R-92-004, July 1992 (revised) Table of Contents Disclaimer Acknowledgements Significant Changes in This Revision Radon and Radon Decay Product Measurement Methods Section 1: General Considerations 1.1 Introduction and Background 1.2 General Guidance on Measurement Strategy, Measurement Conditions, Device Location Selection, and Documentation 1.3 Quality Assurance Section 2: Indoor Radon Measurement Device Protocols 2.1 Protocol for Using Continuous Radon Monitors (CR) to Measure Indoor Radon Concentrations 2.2 Protocol for Using Alpha Track Detectors (AT or ATD) to Measure Indoor Radon Concentrations 2.4 Protocol for Using Activated Charcoal Adsorption Devices (AC) to Measure Indoor Radon Concentrations 2.5 Protocol for Using Charcoal Liquid Scintillation (LS) Devices to Measure Indoor Radon Concentrations 2.6 Protocol for Using Grab Radon Sampling (GB, GC, GS), Pump/Collapsible Bag Devices (PB), and Three-Day Integrating Evacuated Scintillation Cells (SC) to Measure Indoor Radon Concentrations 2.7 Interim Protocol for Using Unfiltered Track Detectors (UT) to Measure Indoor Radon Concentrations Section 3: Indoor Radon Decay Product Measurement Device Protocols 3.1 Protocol for Using Continuous Working Level Monitors (CW) to Measure Indoor Radon Decay Product Concentrations 3.2 Protocol for Using Radon Progeny Integrating Sampling Units (RPISU or RP) to Measure Indoor Radon Decay Product Concentrations 3.3 Protocol for Using Grab Sampling-Working Level (GW) to Measure Indoor Radon Decay Product Concentrations Glossary References Please Note: EPA closed its National Radon Proficiency Program (RPP) in 1998. -

Neutron Sources
Neutron Sources Nadia Fomin Fundamental Neutron Physics Summer School Knoxville, TN 2015 Much content “borrowed” from Kevin Anderson, Mike Snow, Geoff 1 Greene, Scott Dewey, Mike Moncko, Jack Carpenter, and many others http://knoxbeerweek.com/ Neutron Sources Nadia Fomin Fundamental Neutron Physics Summer School Knoxville, TN 2015 Much content “borrowed” from Kevin Anderson, Mike Snow, Geoff 3 Greene, Scott Dewey, Mike Moncko, Jack Carpenter, and many others What is neutron physics ? Research which uses “low energy” neutrons from nuclear reactors and accelerator-driven spallation sources to address questions in nuclear, particle, and astrophysics Neutron Properties -22 Electric charge: qn=0, electrically neutral [qn<10 e] -5 -25 2 Size: rn~10 Angstrom=1 Fermi [area~ 10 cm =0.1 “barn”] Internal Structure: quarks [ddu, md~ mu~few MeV ] + gluons Spin: sn= 1/2 [Fermi statistics] Magnetic Dipole Moment: mn/ mp = -0.68497935(17) -26 Electric Dipole Moment: zero[dn < 10 e-cm] Mass: mn=939.566 MeV [mn> mp+ me, neutrons can decay] Lifetime: tn=880ish (depends on whom you ask) Neutrons are hard to get Neutrons are bound in nuclei, need several MeV for liberation E E=0 We want E~kT~25 meV (room temperature) VNN How to slow down a heavy neutral particle with Mn= Mp ? Lots of collisions… p n [1/2]N=(1 MeV)/(25 meV) for N collisions E 0 E/2 Neutrons are unstable when free->they can’t be accumulated easily Neutrons: Fast and Furious. And slow and gentle . Thermal ~25meV (2200m/s, λT=1.8Å) . Cold 50μeV-25meV . Very cold 2x10-7 - 5x10-5 eV . -

Energy What Is a Nuclear Reaction?
4/16/2016 Option C: Energy C.3 : Nuclear Fusion and Fission What is a nuclear reaction? • A nuclear reaction is any reaction that involves the nucleus. • These reactions change the identity of an atom, as opposed to chemical reactions which only involve valence electrons. 1 4/16/2016 The nucleus • The nucleus is made up of protons and neutrons. • We know what the protons do – they provide an electrostatic attraction to the electrons close… but what about the neutrons? The Neutrons • The major function of the neutrons is to hold the nucleus together. • The neutrons provide a strong nuclear force of attraction within the nucleus, counteracting the repulsion between the positively charged protons. 2 4/16/2016 How is the nucleus held together? • In the 1930’s it was first observed that the mass of an atoms nucleus is less than the sum of the masses of the protons + neutrons…? • Some of the mass of the nucleus is converted into energy to hold the nucleus together. 3 4/16/2016 Mass Defect • The difference in mass of the nucleus and it’s parts is referred to as the mass defect, and the energy (e=mc 2) it provided is called the nuclear binding energy. 4 4/16/2016 Nuclear vs. Chemical Reactions • This nuclear binding energy is released during nuclear reactions (fission & fusion), and is ~1,000,000X greater than the chemical bond energy released during chemical reactions. What makes an isotope radioactive? • Elements are radioactive when their nucleus is unstable. • The stabilizing force of the neutrons is effective for smaller elements, though all elements above lead are radioactive. -

Nuclear Data As the Alternative Information of Decay and Analyzer
Usman Kadir, NuCLEAR Data as The alternative Information of decay and Analyzer NuCLEAR Data as The Alternative Information of Decay and Analyzer Usman Kadir Pusat Pendidikan dan Pelatihan BA TAN Abstract NuCLEAR Data is the name of visual, color-coded computer program of "Chart of Nuclides" or "Segre Chart". The program gives you comprehensive nuclear decay data that include data on alpha, beta, gamma, x-ray decay etc., and comprehensive search by energy, intensity, half life, radiation type or name of nuclide. NuCLEAR Data is able to show visual decay product and couldfunction as Multi Nuclides Identification and Multi Elements Identification Analysis. It is constructed using Visual Basic Compiler. Abstak NuCLEAR Data adalah nama program komputer untuk peta nuklida atau Segre Chart; data tervisual dan dikodekan warna. Program ini memberikan anda data peluruhan nuklir secara komprehensif, yang meliputi peluruhan alpha, beta, gamma, sinar-x dan dapat mencari energi, intensitas, waktu paro, jenis radiasi dan nama nuklida. NuCLEAR Data dapat menampilkan produk peluruhan secara visual dan dapat berfungsi sebagai analisis untuk Identifikasi multi nuklida dan identifikasi unsur-unsur. Program ini dibuat dengan Visual Basic. Chart of the Nuclides The "Chart of the Nuclides" or Segre Chart is shown in a colored mode, including details Chart of the Nuclides is a plot of all known for each nuclide's meta-stable states. nuclides as a function of Z (Atomic number), in the y-axis, and N (number of neutrons), in NuCLEAR for Windows 95/98/2000IXP is a the x-axis. visual Nuclear Data Library. It can be used as a standalone information system. -
Modern Physics to Which He Did This Observation, Usually to Within About 0.1%
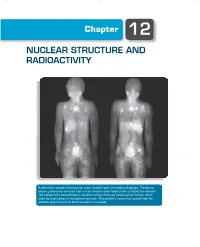
Chapter 12 NUCLEAR STRUCTURE AND RADIOACTIVITY Radioactive isotopes have proven to be valuable tools for medical diagnosis. The photo shows gamma-ray emission from a man who has been treated with a radioactive element. The radioactivity concentrates in locations where there are active cancer tumors, which show as bright areas in the gamma-ray scan. This patient’s cancer has spread from his prostate gland to several other locations in his body. 370 Chapter 12 | Nuclear Structure and Radioactivity The nucleus lies at the center of the atom, occupying only 10−15 of its volume but providing the electrical force that holds the atom together. Within the nucleus there are Z positive charges. To keep these charges from flying apart, the nuclear force must supply an attraction that overcomes their electrical repulsion. This nuclear force is the strongest of the known forces; it provides nuclear binding energies that are millions of times stronger than atomic binding energies. There are many similarities between atomic structure and nuclear structure, which will make our study of the properties of the nucleus somewhat easier. Nuclei are subject to the laws of quantum physics. They have ground and excited states and emit photons in transitions between the excited states. Just like atomic states, nuclear states can be labeled by their angular momentum. There are, however, two major differences between the study of atomic and nuclear properties. In atomic physics, the electrons experience the force provided by an external agent, the nucleus; in nuclear physics, there is no such external agent. In contrast to atomic physics, in which we can often consider the interactions among the electrons as a perturbation to the primary interaction between electrons and nucleus, in nuclear physics the mutual interaction of the nuclear constituents is just what provides the nuclear force, so we cannot treat this complicated many- body problem as a correction to a single-body problem.