PRESCRIBING INFORMATION PENTASPAN* (10% Pentastarch In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blood Products for Neonatal Transfusion
Blood Products for Neonatal Transfusion Transfusion of Red Cell Products A. Red cells for topup transfusion Pedipaks should be used for all red cell topup transfusions in infants. One blood donation is split into four equal volumes (approximately 50ml) and 2 or 4 packs are reserved for an individual baby, depending on weight. The use of pedipaks enables us to minimise patient exposure to multiple donors. Pedipak specifications: • Available in O Positive and O Negative • CMV Negative • Leucocyte Depleted • Suitable for topup transfusion until expiry (42 days from collection) • Commence transfusion within 30 minutes of product receipt Pedipaks and complete transfusion within 4 hours of spiking pack. Volume to be infused: Routine – 15 20ml/Kg over 4 hours. Infants may require IV Frusemide (per RWH Drug Manual) half way through the transfusion – discuss with neonatologist/fellow Emergency – larger volume over shorter time period depending on condition of infant. B. Red cells for Exchange Transfusion Exchange transfusion is generally carried out for hyperbilirubinaemia and/or anaemia usually due to haemolytic disease of the newborn (HDN) or prematurity. ARCBS produces a red cell product specifically for neonatal exchange transfusion. This red cell product has the following specifications: • Group O Negative • Kell negative • CMV negative • Leucocyte Depleted • Fresh (<=5 days) • Known haematocrit (<0.6) • Irradiated at ARCBS (should be transfused within 24 hours of irradiation) • Commence transfusion within 30 minutes of product receipt and complete transfusion within 4 hours of spiking pack. Transfusion of Albumin Volume to be infused: • 4% Albumin as a volume expander. 10 – 20ml/Kg over 30 – 60 minutes • 20% Albumin used for hypoalbuminaemia. -

Clinical Pharmacology of Infusion Fluids
Clinical pharmacology of infusion fluids Robert G. Hahn Linköping University Post Print N.B.: When citing this work, cite the original article. Original Publication: Robert G. Hahn , Clinical pharmacology of infusion fluids, 2012, Acta Medica Lituanica, (19), 3. Licencee: Lithuanian Academy of Sciences http://www.lmaleidykla.lt/ojs/index.php/actamedicalituanica/index Postprint available at: Linköping University Electronic Press http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-91319 ACTA MEDICA LITUANICA. 2012. Vol. 19. No. 3. P. 210–212 © Lietuvos mokslų akademija, 2012 Clinical pharmacology of infusion fluids Robert G. Hahn Fluids are used for intravenous infusion during practically all surgeries, but several different compositions are available on the market. Södertälje Hospital, Crystalloid fluids comprise lactated or acetated Ringer solutions, nor- Södertälje, Sweden; mal saline, Plasma-Lyte, hypertonic saline, and glucose. They lack allergic Anaesthesia and properties but are prone to cause peripheral tissue oedema. Their turn- Intensive Care, over is governed by physiological factors such as dehydration and drug Linköping University, effects. Sweden Colloid fluids include hydroxyethyl starch, albumin, dextran, and gela- tin. These fluids have various degrees of allergic properties and do not promote peripheral oedema. Their half-life is usually about hours. Factors increasing the turnover rate are poorly known but might include inflam- matory states. Current debates include the widespread use of normal saline, which should be replaced by Ringer’s or Plasma-Lyte in most situations, and the kidney damage associated with the use of starch in septic patients. New studies show that hypertonic saline does not improve survival or neuro- logical damage in prehospital care. -

Polymers for Blood Replacement Volume for 6 H, Is 90% Lost in 24 H and Is Said to Lack the Immunological Reactions of from Paul Calvert Dextran
108 Nature Vol. 280 12 July 1979 example, the J/tp at 3.1 GeV and the jet structure survives the final 'colour beneficial in shock cases. High molecular upsilon at 9.4 GeV. In these bound states washing' that creates only colourless weight dextran has anticoagulant activity the heavy quark-antiquark pair can decay hadrons out of the quark and the and it can be used for this purpose after hadronically only by annihilating into antiquark. As each of the gluons from surgery. Low molecular weight dextran is three gluons in much the same way that upsilon decay will on average carry an antigenic but there is no evidence for this positronium disintegrates into three energy of nearly 3 GeV, gluon jets can be with clinically-used dextran, although photons. The gluons of course have to expected there. The three gluons should allergic reactions occasionally occur. evolve into colourless hadrons later. The emerge symmetrically with 120° angles Various dextrans in the molecular weight glueball may lurk among such hadrons. between one another, and there is some range of 40,000-110,000 are used One interesting decay mode is when J/tp preliminary evidence for such events from depending on the balance of effect goes into an energetic photon plus hadrons. PLUTO (CERN Courier 19, 108; 1979). desired. Here the heavy quark-antiquark pair in the Another interesting possible configuration The Chinese work is from the Institute of J/tp annihilate into two gluons and a is one in which an energetic gluon dashes Organic Chemistry in Shanghai, one of the photon. -

The ABC's of Blood Components
The ABC’s of Blood Components Terry Downs, MT(ASCP)SBB Administrative Manager University of Michigan Hospitals Blood Bank and Transfusion Service Objectives Describe three additives used in blood components. List the indications for five blood components. Review whole blood donations versus apheresis collections. Whole Blood Donation Collection of one 450-500 mL of whole blood into a bag Bag then processed into components Additive solutions may be added Takes about 10 minutes to collect 500 mL Apheresis Collection of Blood Whole blood is separated into components during collection Desired component if removed Remaining components are returned to donor Centrifugal technique primarily used in US Allows for “double-red” or multiple plasma Apheresis platelets Granulocytes Collection and Storage Systems Different configurations based on intended processing method Manual processing Automated processing Platelet processing method Approved anticoagulants ACD-A ACD-B CPD CP2D CPDA-1 Contents of Anticoagulant-Preservative Solutions ACD-A CPD CP2D CPDA-1 Trisodium Citrate 22.0 g/L 26.3 g/L 26.3 g/L 26.3 g/L Citric Acid 8.0 g/L 3.27 g/L 3.27 g/L 3.27 g/L Monobasic Sodium Phosphate 0 2.22 g/L 2.22 g/L 2.22 g/L Dextrose 24.5 g/L 25.5 g/L 51.1 g/L 31.9 g/L Adenine 0 0 0 0.275 g/L Shelf Life (days) 21 21 21 35 ACD-A: Anticoagulant citrate-dextrose A CPD: Citrate-phosphate-dextrose CP2D: Citrate-phosphate-dextrose-dextrose CPDA-1: Citrate-phosphate-dextrose-adenine Additive Solutions Extend the shelf life to 42 days. -
Circular of Information for the Use of Human Blood and Blood Components
CIRCULAR OF INFORMATION FOR THE USE OF HUMAN BLOOD Y AND BLOOD COMPONENTS This Circular was prepared jointly by AABB, the AmericanP Red Cross, America’s Blood Centers, and the Armed Ser- vices Blood Program. The Food and Drug Administration recognizes this Circular of Information as an acceptable extension of container labels. CO OT N O Federal Law prohibits dispensing the blood and blood compo- nents describedD in this circular without a prescription. THIS DOCUMENT IS POSTED AT THE REQUEST OF FDA TO PROVIDE A PUBLIC RECORD OF THE CONTENT IN THE OCTOBER 2017 CIRCULAR OF INFORMATION. THIS DOCUMENT IS INTENDED AS A REFERENCE AND PROVIDES: Y • GENERAL INFORMATION ON WHOLE BLOOD AND BLOOD COMPONENTS • INSTRUCTIONS FOR USE • SIDE EFFECTS AND HAZARDS P THIS DOCUMENT DOES NOT SERVE AS AN EXTENSION OF LABELING REQUIRED BY FDA REGUALTIONS AT 21 CFR 606.122. REFER TO THE CIRCULAR OF INFORMATIONO WEB- PAGE AND THE DECEMBER 2O17 FDA GUIDANCE FOR IMPORTANT INFORMATION ON THE CIRCULAR. C T O N O D Table of Contents Notice to All Users . 1 General Information for Whole Blood and All Blood Components . 1 Donors . 1 Y Testing of Donor Blood . 2 Blood and Component Labeling . 3 Instructions for Use . 4 Side Effects and Hazards for Whole Blood and P All Blood Components . 5 Immunologic Complications, Immediate. 5 Immunologic Complications, Delayed. 7 Nonimmunologic Complications . 8 Fatal Transfusion Reactions. O. 11 Red Blood Cell Components . 11 Overview . 11 Components Available . 19 Plasma Components . 23 Overview . 23 Fresh Frozen Plasma . .C . 23 Plasma Frozen Within 24 Hours After Phlebotomy . 28 Components Available . -

Blood Transfusion
BLOOD TRANSFUSION PETER HUDSON CLINICAL SPECIALIST What Are The Risks Associated With Blood Transfusion? • Infection transmission • Hepatitis B • Hepatitis C • HIV • Syphilis • vCJD ? • Transfusion of the wrong blood!!! Sampling Procedure • Step 1: Ask the patient to tell you their: • Full name and date of birth • Check this information against the patient s ID wristband • Get a second independent check when the patient is unconscious / compromised Sampling Procedure • Step 2: Check the patient s ID wristband against documentation e.g. case notes or transfusion request form: • First name • Surname • Date of birth • Hospital number Sampling Procedure • Only bleed one patient at a time • Do NOT use pre-labelled tubes • For transfusion samples hand write the sample tube BEFORE leaving the patients side! • NB: Avoid taking samples from a IV drip arm. • If no alternative stop infusion and wait 15 minutes before taking samples Blood Request Card Mandatory Fields Please Note: • All patients’ requiring blood products will require two group and screen samples to be taken at separate times in order to verify the patient’s correct blood type. Unless there is an existing historical blood group record when an in date second sample will be required. • Or contact blood bank Tel 3746/3747 for advice WHAT DO YOU KNOW ABOUT BLOOD TRANSFUSION? WHAT IS THE AVERAGE VOLUME OF A BAG OF PACKED RED CELLS • 280 MLS • 350 MLS • 450 MLS AVERAGE VOLUME IS 280Mls Approx 450mls is collected from donors Blood is then fractionated into plasma For FFP/cryoprecipitate, -

Crystalloids and Colloids Intravenous Therapy
CRYSTALLOIDS AND COLLOIDS INTRAVENOUS THERAPY NOAH CARPENTER, MD Dr. Noah Carpenter has practiced as a thoracic and peripheral vascular surgeon. He completed the Bachelor of Science in chemistry and medical school and training at the University of Manitoba. Dr. Carpenter completed surgical residency and fellowship at the University of Edmonton and Affiliated Hospitals in Edmonton, Alberta, and an additional Adult Cardiovascular and Thoracic Surgery fellowship at the University of Edinburgh, Scotland. He has specialized in microsurgical techniques, vascular endoscopy, laser and laparoscopic surgery in Brandon, Manitoba and Vancouver Island, British Columbia, Canada and in Colorado, Texas, and California. Dr. Carpenter has an Honorary Doctorate of Law from the University of Calgary, and was appointed a Citizen Ambassador to China, and has served as a member of the Native Physicians Association of Canada, the Canadian College of Health Service Executives, the Science Institute of the Northwest Territories, the Canada Science Council, and the International Society of Endovascular Surgeons, among others. He has been an inspiration to youth, motivating them to understand the importance of achieving higher education. Abstract A majority of hospital patients receive some form of intravenous therapy during a hospital stay, and a significant number of those patients require fluid replacement in the form of volume expanders. There are two types of volume expanders, crystalloids and colloids, and each has advantages and disadvantages. While there continues some controversy in the research over which volume expander is the best choice during specific situations of resuscitation, general medical wisdom exists that allows health clinicians to understand the best use of each and to provide patients with the most effective treatment possible. -

Abuse of Fresh Frozen Plasma
BRITISH MEDICAL JOURNAL VOLUME 295 1 AUGUST 1987 287 high in fibre, without excess caffeine containing drinks is hazardous. Blood, or any of its unpasteurised deriva- Br Med J (Clin Res Ed): first published as 10.1136/bmj.295.6593.287 on 1 August 1987. Downloaded from or alcohol, should keep the patient with true functional tives, may transmit infection, including hepatitis and the hypoglycaemia free ofsymptoms. acquired immune deficiency syndrome. Occasionally, the D J BETTERIDGE antibodies present in plasma may produce harmful effects Senior Lecturer in Medicine and Consultant Endocrinologist, for example, leucoagglutinins may cause pulmonary infil- Rayne Institute, trates.8 Anti-A and anti-B in plasma may destroy the University College London, London WC1E 6JJ recipient's red cells, although this hazard can be avoided by using fresh frozen plasma that is ABO compatible. Fresh I Fischer KF, Lees JA, Newman JH. Hypoglycaemia in hospitalized patients, causes and outcome. frozen plasma may also cause hypersensitivity reactions.8 To N EnglJMed 1986;315: 1245-50. 2 Malouf R, Brust JCM. Hypoglycaemia: causes, neurological manifestations and outcome. take a wider view, any fresh plasma retained at a regional Ann Neurol 1985;17:421-30. transfusion centre and supplied as fresh plasma to hospitals is 3 Marks V, Rose FC. Hypoglycaemia. 2nd ed. Oxford: Blackwell Scientific, 1981. 4 Service FJ. Hypoglycaemic disorders. Boston: G K Hall, 1983. withheld from the national Blood Products Laboratory. In 5 Nelson RL. Hypoglycaemia-fact or fiction. Mayo Clin Proc 1985;60:844-50. is now called 6 Plum F. What causes infarction in ischaemic brain? The Robert Wartenberg lecture. -

PERSONAL CHOICE: Fractions/Treatments/Procedures/Equipment PERSONAL CHOICE: FRACTIONS
Bloodless Care Center/Patient Blood Management (BCC/PBM) Program PERSONAL CHOICE: Fractions/Treatments/Procedures/Equipment PERSONAL CHOICE: FRACTIONS 1. FRACTIONS FROM RED BLOOD CELLS (RBCs): Red Blood Cells (about 40-45% of the blood) transport oxygen from the lungs to body cells. People who have low numbers of red blood cells (RBCs) are said to have anemia. a. HEMOGLOBIN: A protein that helps carry oxygen from the lungs to the rest of the body, and then returns carbon dioxide from the body to the lungs. b. HEMIN: An enzyme inhibitor (salt) derived from the Hemoglobin protein used to treat rare genetic blood disorders (Porphryias). 2. FRACTIONS FROM WHITE BLOOD CELLS (WBCs): White Blood Cells (about 1% of the blood) act as one of the body’s defense against infection (bacteria, viruses). White blood cell transfusions are given rarely. Instead of transfusing WBCs, doctors now commonly use drugs called colony-stimulating factors or growth factors to help the body make its own. a. INTERFERONS/INTERLEUKINS: Small proteins responsible for helping your body to fight infections. Also used in some cancer treatments. 3. FRACTIONS FROM PLATELETS: Platelets (Thrombocytes) are small fragments of cells that prevent blood loss by stopping the bleeding at site of injury (forming clots). At this time, fractions from platelets are not typically used. 4. FRACTIONS FROM PLASMA/Fresh Frozen Plasma (FFP): Plasma/Fresh Frozen Plasma (FFP) is the liquid part of blood and is made of approximately 90% water. Albumin, clotting factors, salts/electrolytes, sugars, fats, vitamins, and hormones from Plasma can be used in medical/surgical care. -

Blood Products
Joint United Kingdom (UK) Blood Transfusion and Tissue PDF Generated JPAC Transplantation Services Professional Advisory Committee 02/10/2021 10:01 Transfusion Handbook 3.3: Blood products http://www.transfusionguidelines.org/transfusion-handbook/3-providing-safe-blood/3-3-blood-products 3.3: Blood products These are classified as blood components prepared in the blood transfusion centre (red cells, platelets, fresh frozen plasma and cryoprecipitate) or plasma derivatives manufactured from pooled plasma donations in plasma fractionation centres (such as albumin, coagulation factors and immunoglobulins). Plasma derivatives are covered by the Medicines Act and, like any other drug, must be prescribed by a licensed practitioner. Since 1999, as a vCJD risk-reduction measure, all plasma derivatives used in the UK are manufactured using donations from countries with a low risk of vCJD. 3.3.1: Blood components Whole blood is now rarely used for transfusion. Blood component therapy makes clinical sense as most patients require a specific element of blood, such as red cells or platelets, and the dose can then be optimised. Each component is stored under ideal conditions (e.g. red cells must be refrigerated, platelets must not) and the use of precious blood donations becomes more efficient. The use of blood components in clinical practice is covered in Chapters 7 to 10. The process of producing blood components and plasma derivatives is summarised in Figure 3.1. 3.3.2: Labelling of blood components 3.3.2.1: Blood component labels The content of blood pack labels attached at the transfusion centre is prescribed by the Blood Safety and Quality Regulations 2005 (BSQR). -
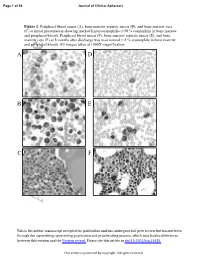
Comparison of Two Leukocytapheresis Protocols in a Case of Idiopathic
Page 1 of 26 Journal of Clinical Apheresis Figure 1. Peripheral blood smear (A), bone marrow aspirate smear (B), and bone marrow core (C) at initial presentation showing marked hypereosinophilia (>90 % eosinophils in bone marrow and peripheral blood). Peripheral blood smear (F), bone marrow aspirate smear (E), and bone marrow core (F) at 8 months after discharge was near normal (<5 % eosinophils in bone marrow and peripheral blood). All images taken at 1000X magnification. A D B E C F Accepted Article This is the author manuscript accepted for publication and has undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version record. Please cite this article as doi:10.1002/jca.21435. This article is protected by copyright. All rights reserved. Journal of Clinical Apheresis Page 2 of 26 Figure 2. Timeline of WBC count and peripheral eosinophil ratio with leukocytapheresis and medications. DVT: deep vein thrombosis, PICC: peripherally inserted central catheter, ANC: absolute neutrophil count Accepted Article John Wiley & Sons This article is protected by copyright. All rights reserved. Page 3 of 26 Journal of Clinical Apheresis Table 1. Summary of diagnostic workup. Select Admission Labs • WBC 225 K/µL, Hgb 10.1 g/dL, Hct 27.4 %, Plt 136 • Eosinophil ratio 96.0 %, Absolute eosinophil 216 K/µL • Troponin 5.06 ng/mL, Lactate dehydrogenase 877 IU/L • Ferritin 680 ng/mL, C-Reactive protein 4.4 mg/dL • D-Dimer 1.73 mg/L Cytogenetic, -

Colloid Starches (Volume Expanders)
TITLE: Hydroxyethyl Starch versus Other Plasma Volume Expanders: A Review of the Clinical and Cost-Effectiveness, and Guidelines for Use DATE: 23 May 2013 CONTEXT AND POLICY ISSUES Fluid resuscitation is indicated for the management of hypovolemia (decreased blood plasma volume) and hypovolemic shock,1 and its ultimate objective is to restore organ perfusion and tissue oxygenation.2 Hypovolemia can be induced by a wide range of clinical conditions such as dehydration, burns, sepsis, malignancies, trauma, hemorrhage, and surgical anesthesia. Clinical signs of hypovolemia include blood pressure, urine output, mental status, and peripheral perfusion.1 There are two main types of fluids used for fluid resuscitation, colloids and crystalloids. Crystalloid solutions include normal saline and balanced fluids such as Ringer’s lactate.3 Human albumin preparations are natural colloids while dextran, gelatin, and starch products are synthetic colloids, also called synthetic plasma volume expanders.3 Hydroxyethyl starches are a common choice for fluid resuscitation.4 They are preferred over albumin because of their relatively lower price.5 These starches are supplied with different molecular weights ranging from 120 kDa to >450kDa. They are also characterized by the degree of substitution which is the molar substitution by hydroxyethyl groups. The degree of substitution ranges from 0.4 to 0.7. It is believed that the molecular weight and degree of substitution can affect patient outcomes.6 Four hydroxyethyl starches are available in Canada; these