Colloid Starches (Volume Expanders)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intravenous And/Or Peripheral Saline Lock Insertion and Maintenance
Policies & Procedures Title: INTRAVENOUS AND/OR PERIPHERAL SALINE LOCK INSERTION AND MAINTENANCE ID Number: 1118 Authorization Source: Nursing Date Revised: September 2013 [X] SHR Nursing Practice Committee Date Effective: May 1999 Date Reaffirmed: May 2015 Scope: SHR & Affiliates Any PRINTED version of this document is only accurate up to the date of printing 22-Jul-15. Saskatoon Health Region (SHR) cannot guarantee the currency or accuracy of any printed policy. Always refer to the Policies and Procedures site for the most current versions of documents in effect. SHR accepts no responsibility for use of this material by any person or organization not associated with SHR. No part of this document may be reproduced in any form for publication without permission of SHR. DEFINITIONS Flushing – Injection of a solution into the intravenous (IV) catheter/cap to prevent mixing of incompatible solutions and clean the catheter of blood or fibrin buildup. Turbulent flush technique – A method of flushing using a “stop-start” technique which “scrubs” the inside of the catheter lumen, preventing the build-up of fibrin or medication residue. 1. PURPOSE 1.1 To minimize the risks of infection and other complications associated with the insertion and maintenance of intravenous catheters. 2. POLICY 2.1 Who may start IV • RN/RPN/GN competent in IV starts (NICU-Level 2 orientated RN only) • Nursing Students under direct RN/GN/RPN/LPN/GPN supervision • LPN/GPN who have successfully completed the IV/Blood Administration Course • Home Intravenous Therapy Program (HITP) – RN’s only 2.2 A prescriber order is required to start an IV except in an emergency situation or for restarts. -

Intravenous Fluids
See also IV fluid guidelines at www.rch.org.au/clinicalguide/cpg.cfm?doc_id=5203, contact email: [email protected] or [email protected] Intravenous fluids Hourly maintenance intravenous fluid requirements [Resuscitation bolus (Normal saline): 10-20 ml/kg] Weight (kg) 4 6 8 10 12 14 16 20 30 40 50 60 70 ml/hr 16 24 32 40 44 48 52 60 70 80 90 100 100 Reminders 1. Check weight of patient 2. Check maintenance fluids infusion rate according to weight. Remember that the maintenance fluid volume will need to be reduced in many unwell children, especially children with meningitis, bronchiolitis, pneumonia, some surgical problems and many children with hyponatraemia. 3. Consider why the patient needs IV fluid. If a child is prescribed anything other than a 0.9% or 0.45% NaCl solution, ask why. 4. Check the written order 5. If in doubt about fluids please ask, call a paediatric registrar, a consultant or the ICU Type of fluid Comment Na+ Cl- K+ Lactate Ca++ Glucose See also Clinical Practice Guidelines Intravenous Fluids (mmol/L) (mmol/L) (mmol/L) (mmol/L) (mmol/L) (gram/L) Monitor 0.9% NaCl Isotonic, contains no glucose 150 150 - - - - Clinical signs of (Normal Saline) Use for initial volume resuscitation, e.g. dehydration and over- septic shock, trauma. hydration 0.9% NaCl with 5% dextrose Use as maintenance fluid for suspected 150 150 50 (Normal saline with glucose) meningitis, acute neurological conditions, Weigh prior to where IV fluids are used for gastroenteritis or commencement of IV when the serum sodium is low. -

Blood Products for Neonatal Transfusion
Blood Products for Neonatal Transfusion Transfusion of Red Cell Products A. Red cells for topup transfusion Pedipaks should be used for all red cell topup transfusions in infants. One blood donation is split into four equal volumes (approximately 50ml) and 2 or 4 packs are reserved for an individual baby, depending on weight. The use of pedipaks enables us to minimise patient exposure to multiple donors. Pedipak specifications: • Available in O Positive and O Negative • CMV Negative • Leucocyte Depleted • Suitable for topup transfusion until expiry (42 days from collection) • Commence transfusion within 30 minutes of product receipt Pedipaks and complete transfusion within 4 hours of spiking pack. Volume to be infused: Routine – 15 20ml/Kg over 4 hours. Infants may require IV Frusemide (per RWH Drug Manual) half way through the transfusion – discuss with neonatologist/fellow Emergency – larger volume over shorter time period depending on condition of infant. B. Red cells for Exchange Transfusion Exchange transfusion is generally carried out for hyperbilirubinaemia and/or anaemia usually due to haemolytic disease of the newborn (HDN) or prematurity. ARCBS produces a red cell product specifically for neonatal exchange transfusion. This red cell product has the following specifications: • Group O Negative • Kell negative • CMV negative • Leucocyte Depleted • Fresh (<=5 days) • Known haematocrit (<0.6) • Irradiated at ARCBS (should be transfused within 24 hours of irradiation) • Commence transfusion within 30 minutes of product receipt and complete transfusion within 4 hours of spiking pack. Transfusion of Albumin Volume to be infused: • 4% Albumin as a volume expander. 10 – 20ml/Kg over 30 – 60 minutes • 20% Albumin used for hypoalbuminaemia. -

Clinical Pharmacology of Infusion Fluids
Clinical pharmacology of infusion fluids Robert G. Hahn Linköping University Post Print N.B.: When citing this work, cite the original article. Original Publication: Robert G. Hahn , Clinical pharmacology of infusion fluids, 2012, Acta Medica Lituanica, (19), 3. Licencee: Lithuanian Academy of Sciences http://www.lmaleidykla.lt/ojs/index.php/actamedicalituanica/index Postprint available at: Linköping University Electronic Press http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-91319 ACTA MEDICA LITUANICA. 2012. Vol. 19. No. 3. P. 210–212 © Lietuvos mokslų akademija, 2012 Clinical pharmacology of infusion fluids Robert G. Hahn Fluids are used for intravenous infusion during practically all surgeries, but several different compositions are available on the market. Södertälje Hospital, Crystalloid fluids comprise lactated or acetated Ringer solutions, nor- Södertälje, Sweden; mal saline, Plasma-Lyte, hypertonic saline, and glucose. They lack allergic Anaesthesia and properties but are prone to cause peripheral tissue oedema. Their turn- Intensive Care, over is governed by physiological factors such as dehydration and drug Linköping University, effects. Sweden Colloid fluids include hydroxyethyl starch, albumin, dextran, and gela- tin. These fluids have various degrees of allergic properties and do not promote peripheral oedema. Their half-life is usually about hours. Factors increasing the turnover rate are poorly known but might include inflam- matory states. Current debates include the widespread use of normal saline, which should be replaced by Ringer’s or Plasma-Lyte in most situations, and the kidney damage associated with the use of starch in septic patients. New studies show that hypertonic saline does not improve survival or neuro- logical damage in prehospital care. -

High Volume Sinonasal Budesonide Irrigations for Chronic Rhinosinusitis
oepidem ac io m lo Rudmik, Adv Pharmacoepidemiol Drug Saf 2014, 3:2 r g a y h & P Advances in Pharmacoepidemiology & DOI: 10.4172/2167-1052.1000148 D n i r u s g e c ISSN: 2167-1052 S n a a f v e t d y A Drug Safety Review Article Open Access High Volume Sinonasal Budesonide Irrigations for Chronic Rhinosinusitis: An Update on the Safety and Effectiveness Luke Rudmik* Division of Otolaryngology–Head and Neck Surgery, Department of Surgery, University of Calgary, Calgary, Alberta, Canada Abstract Chronic rhinosinusitis (CRS) is a common inflammatory disease of the paranasal sinuses associated with severe impairments in patient quality of life, sleep, and productivity. Topical corticosteroid therapy is a key component to a successful management plan for patients with CRS. Delivering topical medical therapies using high-volume sinonasal irrigations are commonly used following endoscopic sinus surgery (ESS) due to its proven efficacy for improving drug delivery into the paranasal sinuses. Topical high volume budesonide irrigations have become a popular off- label management strategy for CRS with the purpose to improve topical steroid delivery into the sinonasal cavities. Early evidence outlined in this review suggests that high volume sinonasal budesonide irrigations are an effective treatment modality in patients with CRS following ESS. Overall it appears that short-term use of this therapy is likely safe, however, future studies will need to assess the safety of higher doses and longer-term therapy of budesonide irrigations in patients with CRS. Keywords: Chronic rhinosinusitis; Sinusitis; Sinonasal; Nasal; mixing an active topical medical agent with an isotonic saline solution Budesonide; Topical Steroid; Safety; Irrigations; Corticosteroid; followed by a low-pressure delivery into the nasal cavity using either a Effectiveness squeeze bottle or neti pot. -

The Effect of Dexamethasone on Chronic Pulmonary Oxygen Toxicity in Infant Mice
003 1-399818912504-0353$02.00/0 PEDIATRIC RESEARCH Vol. 25, No. 4, 1989 Copyright 0 1989 International Pediatric Research Foundation, Inc Printrd in G.S A The Effect of Dexamethasone on Chronic Pulmonary Oxygen Toxicity in Infant Mice N. OHTSU. R. L. ARIAGNO, T. E. SWEENEY, L. DAVIS, L. MOSES, R. PETRICEKS, I. DAEHNE, K. BENSCH, AND W. H. NORTHWAY, JK. De~~urtmcntsofRadiology, Pathology, Pediatrics, Stanfird University Medical Center, Stanbrd, Califhrnia 94305 ABSTRACT. The effect of dexamethasone (0.1, 1, and 5 ported in the clinical studies of Mammel et al. (1, 2) and Avery mg/kg/d given subcutaneously from d 14-18) was tested in et al. (3). infant mice continuously exposed from birth to either hu- midified air or 80% oxygen. Dexamethasone significantly decreased lung wet wt (p < 0.01), lung water (p < 0.021), METHODS lung dry wt, protein, and DNA (p < 0.001) in both air- Animal selection, environment, and drug administrution. In and oxygen-exposed animals. Dexamethasone, however, each experiment, two litters of naturally born C57BL mice were had no effect on lung compliance measured after animals weighed and sex identified within 24 h of birth. Natural litter were killed on d 18. It also had no effect on the increase in size was reduced to five by selecting the five animals with the the blood-air barrier thickness or decrease in the blood-air highest birth wt. Each of the five newborn mice was marked and exchange surface area seen in the 80% oxygen-exposed assigned a serial number which determined the treatment assign- mice. -

Maintenance Fluid Therapy with Saline, Dextrose-Supplemented Saline Or Lactated Ringer in Childhood: Short-Term Metabolic Effect
nutrients Article Maintenance Fluid Therapy with Saline, Dextrose-Supplemented Saline or Lactated Ringer in Childhood: Short-Term Metabolic Effects 1, 1,2,3, , 1 1 Alessandra Ricciuti y, Gregorio P. Milani * y , Silvia Tarantino , Roberta Ghilardi , Sebastiano A.G. Lava 4, Marco Alberzoni 2, Mario G. Bianchetti 5 and Carlo Agostoni 1,2,3 1 Pediatric Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy; [email protected] (A.R.); [email protected] (S.T.); [email protected] (R.G.); [email protected] (C.A.) 2 Department of Clinical Sciences and Community Health, Università degli Studi di Milano, 20122 Milan, Italy; [email protected] 3 Italian Society for Pediatric Gastroenterology Hepatology and Nutrition (SIGENP), 20126 Milan, Italy 4 Pediatric Cardiology Unit, Department of Pediatrics, Centre Hospitalier Universitaire Vaudois, and University of Lausanne, 1010 Lausanne, Switzerland; [email protected] 5 Università della Svizzera Italiana, 6900 Lugano, Switzerland; [email protected] * Correspondence: [email protected]; Tel.: +39-025-503-2266 These two authors equally contributed to the study. y Received: 29 March 2020; Accepted: 14 May 2020; Published: 17 May 2020 Abstract: Maintenance with isotonic fluids is recommended in children with gastroenteritis and failure of oral rehydration therapy. However, little is known on the short-term effects of the commonly prescribed intravenous solutions on metabolic balance in children. The aim of this study is to report on our experience with normal saline, dextrose-supplemented saline and lactated Ringer solution. Methods: A retrospective analysis from the charts of all previously apparently healthy children with acute gastroenteritis, mild to moderate dehydration and failure of oral rehydration, evaluated between January 2016 and December 2019 at our institution, was performed. -
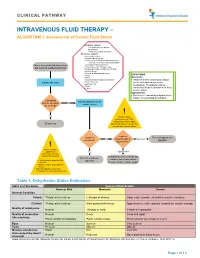
Intravenous Fluid Therapy – Algorithm 1
CLINICAL PATHWAY INTRAVENOUS FLUID THERAPY – ALGORITHM 1. Assessment of Overall Fluid Status Inclusion criteria: • All inpatients except those listed below • Patients pending admission Exclusion criteria: •Acute kidney injury •Chronic kidney disease •Endocrine or renal abnormalities leading to electrolyte derangements including DKA Patient who meets inclusion criteria •Oncology treatment protocol •Patients less than 30 days of age, and warrants supplemental fluids Including premature infants corrected for gestational age •Increased intracranial pressure •PICU DEFINITIONS •NICU Euvolemic: •Total Parenteral Nutrition dependent • Patient is at their ideal volume status Obtain vital signs •Pyloric Stenosis (neither dehydrated nor volume •Burn patients overloaded). The patient requires •Shock •Codes intravenous fluids to maintain their ideal volume status. Hypovolemic: • Patient is at least mildly dehydrated (see Table 1 for estimating dehydration) Can patient Assess patient’s current tolerate adequate No volume status enteral fluids? ! Yes Volume status assessment is 100% clinical. Do not rely upon Off pathway laboratory values to determine the patient’s volume status. Is patient Is patient Refer to hypovolemic No hypervolemic or Hypovolemic euvolemic? algorithm hypovolemic? ! Prior to starting a patient on Yes Hypervolemic maintenance IV fluids, consider the following: Reassess need for IV fluids and Refer to euvolemic consider issues with oncotic • Risk factors for abnormal ADH algorithm secretion pressure and/or cardiac output • Initial -

Polymers for Blood Replacement Volume for 6 H, Is 90% Lost in 24 H and Is Said to Lack the Immunological Reactions of from Paul Calvert Dextran
108 Nature Vol. 280 12 July 1979 example, the J/tp at 3.1 GeV and the jet structure survives the final 'colour beneficial in shock cases. High molecular upsilon at 9.4 GeV. In these bound states washing' that creates only colourless weight dextran has anticoagulant activity the heavy quark-antiquark pair can decay hadrons out of the quark and the and it can be used for this purpose after hadronically only by annihilating into antiquark. As each of the gluons from surgery. Low molecular weight dextran is three gluons in much the same way that upsilon decay will on average carry an antigenic but there is no evidence for this positronium disintegrates into three energy of nearly 3 GeV, gluon jets can be with clinically-used dextran, although photons. The gluons of course have to expected there. The three gluons should allergic reactions occasionally occur. evolve into colourless hadrons later. The emerge symmetrically with 120° angles Various dextrans in the molecular weight glueball may lurk among such hadrons. between one another, and there is some range of 40,000-110,000 are used One interesting decay mode is when J/tp preliminary evidence for such events from depending on the balance of effect goes into an energetic photon plus hadrons. PLUTO (CERN Courier 19, 108; 1979). desired. Here the heavy quark-antiquark pair in the Another interesting possible configuration The Chinese work is from the Institute of J/tp annihilate into two gluons and a is one in which an energetic gluon dashes Organic Chemistry in Shanghai, one of the photon. -

The ABC's of Blood Components
The ABC’s of Blood Components Terry Downs, MT(ASCP)SBB Administrative Manager University of Michigan Hospitals Blood Bank and Transfusion Service Objectives Describe three additives used in blood components. List the indications for five blood components. Review whole blood donations versus apheresis collections. Whole Blood Donation Collection of one 450-500 mL of whole blood into a bag Bag then processed into components Additive solutions may be added Takes about 10 minutes to collect 500 mL Apheresis Collection of Blood Whole blood is separated into components during collection Desired component if removed Remaining components are returned to donor Centrifugal technique primarily used in US Allows for “double-red” or multiple plasma Apheresis platelets Granulocytes Collection and Storage Systems Different configurations based on intended processing method Manual processing Automated processing Platelet processing method Approved anticoagulants ACD-A ACD-B CPD CP2D CPDA-1 Contents of Anticoagulant-Preservative Solutions ACD-A CPD CP2D CPDA-1 Trisodium Citrate 22.0 g/L 26.3 g/L 26.3 g/L 26.3 g/L Citric Acid 8.0 g/L 3.27 g/L 3.27 g/L 3.27 g/L Monobasic Sodium Phosphate 0 2.22 g/L 2.22 g/L 2.22 g/L Dextrose 24.5 g/L 25.5 g/L 51.1 g/L 31.9 g/L Adenine 0 0 0 0.275 g/L Shelf Life (days) 21 21 21 35 ACD-A: Anticoagulant citrate-dextrose A CPD: Citrate-phosphate-dextrose CP2D: Citrate-phosphate-dextrose-dextrose CPDA-1: Citrate-phosphate-dextrose-adenine Additive Solutions Extend the shelf life to 42 days. -
IDF Guide for Nurses Imimmmunnoogglolboubliun Ltinhe Trahpeyr Faopr Y Fopr Rpirmimaarryy I Mimmumnoudneoficdieenfciyc Ideisnecasy Es Diseases IDF GUIDE for NURSES
IDF Guide for Nurses ImImmmunnoogglolboubliun lTinhe Trahpeyr faopr y foPr rPirmimaarryy I mImmumnoudneoficdieenfciyc iDeisnecasy es Diseases IDF GUIDE FOR NURSES IMMUNOGLOBULIN THERAPY FOR PRIMARY IMMUNODEFICIENCY DISEASES THIRD EDITION COPYRIGHTS 2004, 2007, 2012 IMMUNE DEFICIENCY FOUNDATION Copyright 2012 by the Immune Deficiency Foundation, USA. PRINT: 12/2013 Readers may redistribute this publication to other individuals for non-commercial use, provided that the text, html codes, and this notice remain intact and unaltered in any way. The IDF Guide for Nurses may not be resold, reprinted or redistributed for compensation of any kind without prior written permission from the Immune Deficiency Foundation. If you have any questions about permission, please contact: Immune Deficiency Foundation, 40 West Chesapeake Avenue, Suite 308, Towson, MD 21204, USA, or by telephone: 800.296.4433. This publication has been made possible through a generous grant from IDF Guide for Nurses Immunoglobulin Therapy for Primary Immunodeficiency Diseases Third Edition Immune Deficiency Foundation 40 West Chesapeake Avenue, Suite 308 Towson, MD 21204 800.296.4433 www.primaryimmune.org Editor: M. Elizabeth M. Younger CRNP, PhD Johns Hopkins, Baltimore, Maryland Vice Chair, Immune Deficiency Foundation Nurse Advisory Committee Associate Editors: Rebecca H. Buckley, MD Duke University School of Medicine, Durham, NC Chair, Immune Deficiency Foundation Medical Advisory Committee Christine M. Belser Immune Deficiency Foundation, Towson, Maryland Kara Moran Immune Deficiency -

Two Doses of Early Intravenous Dexamethasone for the Prevention of Bronchopulmonary Dysplasia in Babies with Respiratory Distress Syndrome1
0031-3998/9413601-0122$03.00/0 PEDIATRIC RESEARCH Vol. 36, No. 1, 1994 Copyright 0 1994 International Pediatric Research Foundation, Inc. Printed in (I.S.A. Two Doses of Early Intravenous Dexamethasone for the Prevention of Bronchopulmonary Dysplasia in Babies with Respiratory Distress syndrome1 RAYMOND J. SANDERS,' CHRISTOPHER COX, DALE L. PHELPS, AND ROBERT A. SINKIN Departments of Pediatrics (Neonatology)[R. J. S., D. L. P., R.A. S. 1 and Biostatistics [C.C. I, Strong Children S Medical Center, Rochester, New York 14642 Bronchopulmonary dysplasia is an important complica- methasone group was 89% versus 67% in the placebo group tion of ventilation in babies for which treatment with ste- @ = 0.08, XZ analysis). Survival without bronchopulmo- roids has been advocated. We report the results of a phase nary dysplasia, diagnosed at 36 wk corrected gestational I study of early i.v. dexamethasone to prevent the devel- age, was 68% in the dexamethasone group versus 43% in opment of bronchopulmonary dysplasia in a high-risk pop- the placebo group @ = 0.14). Mean blood pressure was ulation of ventilated premature babies, < 30 wk gestation, elevated on study d 4 through 7 in the dexamethasone with surfactant-treated respiratory distress syndrome. This group, but this difference resolved by study d 10 without study used a limited dexamethasone dosing regimen to pharmacologic intervention. No differences in hyperglyce- minimize toxicity but used administration early in the mia, incidence of intraventricular hemorrhage (or its sever- course of acute lung disease to interrupt the injury cycle. ity), or days to regain birth weight were seen.