High Volume Sinonasal Budesonide Irrigations for Chronic Rhinosinusitis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intravenous And/Or Peripheral Saline Lock Insertion and Maintenance
Policies & Procedures Title: INTRAVENOUS AND/OR PERIPHERAL SALINE LOCK INSERTION AND MAINTENANCE ID Number: 1118 Authorization Source: Nursing Date Revised: September 2013 [X] SHR Nursing Practice Committee Date Effective: May 1999 Date Reaffirmed: May 2015 Scope: SHR & Affiliates Any PRINTED version of this document is only accurate up to the date of printing 22-Jul-15. Saskatoon Health Region (SHR) cannot guarantee the currency or accuracy of any printed policy. Always refer to the Policies and Procedures site for the most current versions of documents in effect. SHR accepts no responsibility for use of this material by any person or organization not associated with SHR. No part of this document may be reproduced in any form for publication without permission of SHR. DEFINITIONS Flushing – Injection of a solution into the intravenous (IV) catheter/cap to prevent mixing of incompatible solutions and clean the catheter of blood or fibrin buildup. Turbulent flush technique – A method of flushing using a “stop-start” technique which “scrubs” the inside of the catheter lumen, preventing the build-up of fibrin or medication residue. 1. PURPOSE 1.1 To minimize the risks of infection and other complications associated with the insertion and maintenance of intravenous catheters. 2. POLICY 2.1 Who may start IV • RN/RPN/GN competent in IV starts (NICU-Level 2 orientated RN only) • Nursing Students under direct RN/GN/RPN/LPN/GPN supervision • LPN/GPN who have successfully completed the IV/Blood Administration Course • Home Intravenous Therapy Program (HITP) – RN’s only 2.2 A prescriber order is required to start an IV except in an emergency situation or for restarts. -

Saline Irrigation for Chronic Rhinosinusitis (Review)
Cochrane Database of Systematic Reviews Saline irrigation for chronic rhinosinusitis (Review) Chong LY, Head K, Hopkins C, Philpott C, Glew S, Scadding G, Burton MJ, Schilder AGM Chong LY, Head K, Hopkins C, Philpott C, Glew S, Scadding G, Burton MJ, Schilder AGM. Saline irrigation for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No.: CD011995. DOI: 10.1002/14651858.CD011995.pub2. www.cochranelibrary.com Saline irrigation for chronic rhinosinusitis (Review) Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 SUMMARY OF FINDINGS FOR THE MAIN COMPARISON . ..... 4 BACKGROUND .................................... 7 OBJECTIVES ..................................... 8 METHODS ...................................... 8 RESULTS....................................... 12 Figure1. ..................................... 14 Figure2. ..................................... 17 Figure3. ..................................... 18 ADDITIONALSUMMARYOFFINDINGS . 22 DISCUSSION ..................................... 25 AUTHORS’CONCLUSIONS . 26 ACKNOWLEDGEMENTS . 27 REFERENCES ..................................... 27 CHARACTERISTICSOFSTUDIES . 31 DATAANDANALYSES. 42 Analysis 1.1. Comparison 1 Nasal saline (hypertonic, 2%, large-volume) versus usual treatment, Outcome 1 Disease- specific HRQL - measured using RSDI (range 0 to 100). ........ 43 Analysis 1.2. Comparison -

Nasal Hygiene
Nasal Hygiene This brochure will guide you through the steps to be followed in order to adequately perform nasal hygiene on your child and will answer any questions you might have. Why perform nasal hygiene? The nose helps you filter, humidify and warm up the air you breathe. In order to fulfill its role this “filter” should be clean! A congested nose prevents the child from easily breathing and can hinder his/her sleep and alimentation. Did you know? Children produce on average one liter (4 cups) of nasal secretions every day and even more when they suffer from colds or respiratory allergies (pollen, dust, etc.) It is not easy for children to take care of these secretions when they cannot efficiently blow their nose. Thus, they need the support of their parents to help them breathe properly. In Canada, children suffer from approximately 6 to 8 colds each year, specially between the months of October and May. Each cold can develop into an ear infection (otitis), sinus infection (sinusitis), chronic cough or breathing problems. As well, 20% of children suffer 3 from allergic rhinitis* (nasal congestion with clear mucus, dry cough, sneezing with nose and throat itching or tingling). Regular nasal hygiene with a saline solution will eliminate secre- tions and small particles (dust, pollen, animal dander, etc.). This will diminish congestion, humidify the nose and prevent nosebleeds. It will also promote: ◗ Better feeding and better sleep; ◗ Less colds and colds of shorter duration; ◗ Less otitis, sinusitis and coughs; ◗ Less use of antibiotics; ◗ For children suffering from asthma, a better control of their condition; ◗ Less absences from day care or school for the children and from work for their parents. -

Intravenous Fluids
See also IV fluid guidelines at www.rch.org.au/clinicalguide/cpg.cfm?doc_id=5203, contact email: [email protected] or [email protected] Intravenous fluids Hourly maintenance intravenous fluid requirements [Resuscitation bolus (Normal saline): 10-20 ml/kg] Weight (kg) 4 6 8 10 12 14 16 20 30 40 50 60 70 ml/hr 16 24 32 40 44 48 52 60 70 80 90 100 100 Reminders 1. Check weight of patient 2. Check maintenance fluids infusion rate according to weight. Remember that the maintenance fluid volume will need to be reduced in many unwell children, especially children with meningitis, bronchiolitis, pneumonia, some surgical problems and many children with hyponatraemia. 3. Consider why the patient needs IV fluid. If a child is prescribed anything other than a 0.9% or 0.45% NaCl solution, ask why. 4. Check the written order 5. If in doubt about fluids please ask, call a paediatric registrar, a consultant or the ICU Type of fluid Comment Na+ Cl- K+ Lactate Ca++ Glucose See also Clinical Practice Guidelines Intravenous Fluids (mmol/L) (mmol/L) (mmol/L) (mmol/L) (mmol/L) (gram/L) Monitor 0.9% NaCl Isotonic, contains no glucose 150 150 - - - - Clinical signs of (Normal Saline) Use for initial volume resuscitation, e.g. dehydration and over- septic shock, trauma. hydration 0.9% NaCl with 5% dextrose Use as maintenance fluid for suspected 150 150 50 (Normal saline with glucose) meningitis, acute neurological conditions, Weigh prior to where IV fluids are used for gastroenteritis or commencement of IV when the serum sodium is low. -

Topical Management of Chronic Rhinosinusitis
Open Access Advanced Treatments in ENT Disorders Review Article Topical Management of chronic rhinosinusitis - A literature review ISSN 1 2 2640-2777 Aremu Shuaib Kayode * and Tesleem Olayinka Orewole 1ENT Department, Federal Teaching Hospital, Ido-Ekiti/Afe Babalola University, Ado Ekiti, Nigeria 2Department of Anaesthesia, Federal Teaching Hospital, Ido Ekiti/Afe Babalola University, Ado Ekiti, Nigeria *Address for Correspondence: Dr. Aremu Shuaib Introduction Kayode, ENT Department, Federal Teaching Chronic rhinosinusitis (CRS) is an inlammatory condition involving nasal passages Hospital, Ido-Ekiti/Afe Babalola University, Ado Ekiti, Nigeria, Tel: +2348033583842; and the paranasal sinuses for 12 weeks or longer [1]. It can be subdivided into three Email: [email protected] types: CRS with nasal polyposis (CRS with NP), CRS without nasal polyposis (CRS Submitted: 08 April 2019 without NP), and Allergic fungal rhinosinusitis (AFRS). To diagnose CRS we require Approved: 25 April 2019 at least two of four of its cardinal signs/symptoms (nasal obstruction, mucopurulent Published: 26 April 2019 discharge, facial pain/pressure, and decreased sense of smell). In addition, direct Copyright: © 2019 Aremu SK, et al. This is visualization or imaging for objective documentation of mucosal inlammation is an open access article distributed under the required. CRS therapy is aimed to reduce its symptoms and improve quality of life as it Creative Commons Attribution License, which cannot be cured in most patients. Thus, the goals of its therapy include the following: permits unrestricted use, distribution, and reproduction in any medium, provided the 1: Control mucosal edema and inlammation of nasal and paranasal sinuses original work is properly cited 2: Maintain adequate sinus ventilation and drainage 3: Treat any infecting or colonizing micro-organisms, if present 4: Reduce the number of acute exacerbations Mucosal remodeling is the most likely underlying mechanism causing irreversible chronic sinus disease, similar to that occur in severe asthma. -

Clinical Pharmacology of Infusion Fluids
Clinical pharmacology of infusion fluids Robert G. Hahn Linköping University Post Print N.B.: When citing this work, cite the original article. Original Publication: Robert G. Hahn , Clinical pharmacology of infusion fluids, 2012, Acta Medica Lituanica, (19), 3. Licencee: Lithuanian Academy of Sciences http://www.lmaleidykla.lt/ojs/index.php/actamedicalituanica/index Postprint available at: Linköping University Electronic Press http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-91319 ACTA MEDICA LITUANICA. 2012. Vol. 19. No. 3. P. 210–212 © Lietuvos mokslų akademija, 2012 Clinical pharmacology of infusion fluids Robert G. Hahn Fluids are used for intravenous infusion during practically all surgeries, but several different compositions are available on the market. Södertälje Hospital, Crystalloid fluids comprise lactated or acetated Ringer solutions, nor- Södertälje, Sweden; mal saline, Plasma-Lyte, hypertonic saline, and glucose. They lack allergic Anaesthesia and properties but are prone to cause peripheral tissue oedema. Their turn- Intensive Care, over is governed by physiological factors such as dehydration and drug Linköping University, effects. Sweden Colloid fluids include hydroxyethyl starch, albumin, dextran, and gela- tin. These fluids have various degrees of allergic properties and do not promote peripheral oedema. Their half-life is usually about hours. Factors increasing the turnover rate are poorly known but might include inflam- matory states. Current debates include the widespread use of normal saline, which should be replaced by Ringer’s or Plasma-Lyte in most situations, and the kidney damage associated with the use of starch in septic patients. New studies show that hypertonic saline does not improve survival or neuro- logical damage in prehospital care. -

Nasal Saline Irrigations for the Symptoms of Chronic Rhinosinusitis (Review)
Nasal saline irrigations for the symptoms of chronic rhinosinusitis (Review) Harvey R, Hannan SA, Badia L, Scadding G This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2009, Issue 1 http://www.thecochranelibrary.com Nasal saline irrigations for the symptoms of chronic rhinosinusitis (Review) Copyright © 2009 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 4 METHODS ...................................... 4 RESULTS....................................... 8 Figure1. ..................................... 9 DISCUSSION ..................................... 13 AUTHORS’CONCLUSIONS . 13 REFERENCES ..................................... 14 CHARACTERISTICSOFSTUDIES . 17 DATAANDANALYSES. 32 Analysis 1.1. Comparison 1 A: Comparison of saline versus no treatment, Outcome 1 Symptom scores. 33 Analysis 1.2. Comparison 1 A: Comparison of saline versus no treatment, Outcome 2 Quality of Life scores (disease specific). ................................... 34 Analysis 1.3. Comparison 1 A: Comparison of saline versus no treatment, Outcome 3 Quality of Life scores (general). 34 Analysis 2.1. Comparison 2 B: Comparison of saline versus ’placebo’, Outcome 1 Quality of Life scores (disease specific) Bulb..................................... -

The Effectiveness of Budesonide Nasal Irrigation After Endoscopic Sinus Surgery in Chronic Rhinosinusitis with Asthma
Clinical and Experimental Otorhinolaryngology Vol. 10, No. 1: 91-96, March 2017 https://doi.org/10.21053/ceo.2016.00220 pISSN 1976-8710 eISSN 2005-0720 Original Article The Effectiveness of Budesonide Nasal Irrigation After Endoscopic Sinus Surgery in Chronic Rhinosinusitis With Asthma Tae Wook Kang·Jae Ho Chung·Seok Hyun Cho·Seung Hwan Lee·Kyung Rae Kim·Jin Hyeok Jeong Department of Otolaryngology-Head and Neck Surgery, Hanyang University School of Medicine, Seoul, Korea Objectives. Budesonide nasal irrigation was introduced recently for postoperative management of patients with chronic rh- inosinusitis. The safety and therapeutic effectiveness of this procedure is becoming accepted by many physicians. The objective of this study was to evaluate the efficacy of postoperative steroid irrigation in patients with chronic rhinosi- nusitis and asthma. Methods. This prospective study involved 12 chronic rhinosinusitis patients with nasal polyps and asthma who received oral steroid treatment for recurring or worsening disease. The 22-item Sinonasal Outcomes Test (SNOT-22) and Lund- Kennedy endoscopy scores were checked before nasal budesonide irrigation, and 1, 2, 4, and 6 months after irriga- tion. We also calculated the total amount of oral steroids and inhaled steroids in the 6 months before irrigation and the 6 months after it. Results. The mean SNOT-22 score improved from 30.8±14.4 before irrigation to 14.2±8.7 after 6 months of irrigation (P=0.030). The endoscopy score also improved from 7.4±4.7 before irrigation to 2.2±2.7 after 6 months (P<0.001). The total amount of oral steroid was decreased from 397.8±97.6 mg over the 6 months before irrigation to 72.7± 99.7 mg over the 6 months after irrigation (P<0.001). -

The Effect of Dexamethasone on Chronic Pulmonary Oxygen Toxicity in Infant Mice
003 1-399818912504-0353$02.00/0 PEDIATRIC RESEARCH Vol. 25, No. 4, 1989 Copyright 0 1989 International Pediatric Research Foundation, Inc Printrd in G.S A The Effect of Dexamethasone on Chronic Pulmonary Oxygen Toxicity in Infant Mice N. OHTSU. R. L. ARIAGNO, T. E. SWEENEY, L. DAVIS, L. MOSES, R. PETRICEKS, I. DAEHNE, K. BENSCH, AND W. H. NORTHWAY, JK. De~~urtmcntsofRadiology, Pathology, Pediatrics, Stanfird University Medical Center, Stanbrd, Califhrnia 94305 ABSTRACT. The effect of dexamethasone (0.1, 1, and 5 ported in the clinical studies of Mammel et al. (1, 2) and Avery mg/kg/d given subcutaneously from d 14-18) was tested in et al. (3). infant mice continuously exposed from birth to either hu- midified air or 80% oxygen. Dexamethasone significantly decreased lung wet wt (p < 0.01), lung water (p < 0.021), METHODS lung dry wt, protein, and DNA (p < 0.001) in both air- Animal selection, environment, and drug administrution. In and oxygen-exposed animals. Dexamethasone, however, each experiment, two litters of naturally born C57BL mice were had no effect on lung compliance measured after animals weighed and sex identified within 24 h of birth. Natural litter were killed on d 18. It also had no effect on the increase in size was reduced to five by selecting the five animals with the the blood-air barrier thickness or decrease in the blood-air highest birth wt. Each of the five newborn mice was marked and exchange surface area seen in the 80% oxygen-exposed assigned a serial number which determined the treatment assign- mice. -

Maintenance Fluid Therapy with Saline, Dextrose-Supplemented Saline Or Lactated Ringer in Childhood: Short-Term Metabolic Effect
nutrients Article Maintenance Fluid Therapy with Saline, Dextrose-Supplemented Saline or Lactated Ringer in Childhood: Short-Term Metabolic Effects 1, 1,2,3, , 1 1 Alessandra Ricciuti y, Gregorio P. Milani * y , Silvia Tarantino , Roberta Ghilardi , Sebastiano A.G. Lava 4, Marco Alberzoni 2, Mario G. Bianchetti 5 and Carlo Agostoni 1,2,3 1 Pediatric Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy; [email protected] (A.R.); [email protected] (S.T.); [email protected] (R.G.); [email protected] (C.A.) 2 Department of Clinical Sciences and Community Health, Università degli Studi di Milano, 20122 Milan, Italy; [email protected] 3 Italian Society for Pediatric Gastroenterology Hepatology and Nutrition (SIGENP), 20126 Milan, Italy 4 Pediatric Cardiology Unit, Department of Pediatrics, Centre Hospitalier Universitaire Vaudois, and University of Lausanne, 1010 Lausanne, Switzerland; [email protected] 5 Università della Svizzera Italiana, 6900 Lugano, Switzerland; [email protected] * Correspondence: [email protected]; Tel.: +39-025-503-2266 These two authors equally contributed to the study. y Received: 29 March 2020; Accepted: 14 May 2020; Published: 17 May 2020 Abstract: Maintenance with isotonic fluids is recommended in children with gastroenteritis and failure of oral rehydration therapy. However, little is known on the short-term effects of the commonly prescribed intravenous solutions on metabolic balance in children. The aim of this study is to report on our experience with normal saline, dextrose-supplemented saline and lactated Ringer solution. Methods: A retrospective analysis from the charts of all previously apparently healthy children with acute gastroenteritis, mild to moderate dehydration and failure of oral rehydration, evaluated between January 2016 and December 2019 at our institution, was performed. -

Isotonic Saline Nasal Irrigation in Clinical Practice: a Literature Review
ISSN 0103-5150 Fisioter. Mov., Curitiba, v. 30, n. 3, p. 639-649, Jul./Sep. 2017 Licenciado sob uma Licença Creative Commons DOI: http://dx.doi.org/10.1590/1980-5918.030.003.AR04 Isotonic saline nasal irrigation in clinical practice: a literature review O uso da ducha nasal com solução salina na prática clínica: uma revisão da literatura Sabrina Costa Lima [a], Ana Carolina Campos Ferreira [b], Tereza Cristina da Silva Brant [b]* [a] Faculdade de Ciências Médicas de Minas Gerais (FCM-MG), Belo Horizonte, MG, Brazil [b] [b] Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil [R] Abstract Introduction: Nasal instillation of saline solution has been used as part of the treatment of patients with upper respiratory tract diseases. Despite its use for a number of years, factors such as the amount of saline solution to be used, degree of salinity, method and frequency of application have yet to be fully explained. Objective: Review the reported outcomes of saline nasal irrigation in adults with allergic rhinitis, acute or chronic sinusitis and after functional endoscopic sinus surgery (FESS), and provide evidence to assist phys- iotherapists in decision making in clinical practice. Methods: A search was conducted of the Pubmed and Cochrane Library databases between 2007 and 2014. A combination of the following descriptors was used as a search strategy: nasal irrigation, nasal lavage, rhinitis, sinusitis, saline, saline solution. Results: Eight clinical trials were included, analyzed according to participant diagnosis. Conclusion: The evidence found was heterogeneous, but contributed to elucidating uncertainties regarding the use of nasal lavage in the clinical practice of physical therapy, such as the protocols used. -
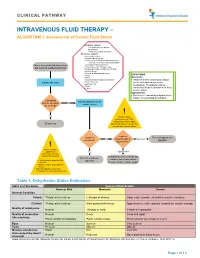
Intravenous Fluid Therapy – Algorithm 1
CLINICAL PATHWAY INTRAVENOUS FLUID THERAPY – ALGORITHM 1. Assessment of Overall Fluid Status Inclusion criteria: • All inpatients except those listed below • Patients pending admission Exclusion criteria: •Acute kidney injury •Chronic kidney disease •Endocrine or renal abnormalities leading to electrolyte derangements including DKA Patient who meets inclusion criteria •Oncology treatment protocol •Patients less than 30 days of age, and warrants supplemental fluids Including premature infants corrected for gestational age •Increased intracranial pressure •PICU DEFINITIONS •NICU Euvolemic: •Total Parenteral Nutrition dependent • Patient is at their ideal volume status Obtain vital signs •Pyloric Stenosis (neither dehydrated nor volume •Burn patients overloaded). The patient requires •Shock •Codes intravenous fluids to maintain their ideal volume status. Hypovolemic: • Patient is at least mildly dehydrated (see Table 1 for estimating dehydration) Can patient Assess patient’s current tolerate adequate No volume status enteral fluids? ! Yes Volume status assessment is 100% clinical. Do not rely upon Off pathway laboratory values to determine the patient’s volume status. Is patient Is patient Refer to hypovolemic No hypervolemic or Hypovolemic euvolemic? algorithm hypovolemic? ! Prior to starting a patient on Yes Hypervolemic maintenance IV fluids, consider the following: Reassess need for IV fluids and Refer to euvolemic consider issues with oncotic • Risk factors for abnormal ADH algorithm secretion pressure and/or cardiac output • Initial