The Effect of Dexamethasone on Chronic Pulmonary Oxygen Toxicity in Infant Mice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intravenous And/Or Peripheral Saline Lock Insertion and Maintenance
Policies & Procedures Title: INTRAVENOUS AND/OR PERIPHERAL SALINE LOCK INSERTION AND MAINTENANCE ID Number: 1118 Authorization Source: Nursing Date Revised: September 2013 [X] SHR Nursing Practice Committee Date Effective: May 1999 Date Reaffirmed: May 2015 Scope: SHR & Affiliates Any PRINTED version of this document is only accurate up to the date of printing 22-Jul-15. Saskatoon Health Region (SHR) cannot guarantee the currency or accuracy of any printed policy. Always refer to the Policies and Procedures site for the most current versions of documents in effect. SHR accepts no responsibility for use of this material by any person or organization not associated with SHR. No part of this document may be reproduced in any form for publication without permission of SHR. DEFINITIONS Flushing – Injection of a solution into the intravenous (IV) catheter/cap to prevent mixing of incompatible solutions and clean the catheter of blood or fibrin buildup. Turbulent flush technique – A method of flushing using a “stop-start” technique which “scrubs” the inside of the catheter lumen, preventing the build-up of fibrin or medication residue. 1. PURPOSE 1.1 To minimize the risks of infection and other complications associated with the insertion and maintenance of intravenous catheters. 2. POLICY 2.1 Who may start IV • RN/RPN/GN competent in IV starts (NICU-Level 2 orientated RN only) • Nursing Students under direct RN/GN/RPN/LPN/GPN supervision • LPN/GPN who have successfully completed the IV/Blood Administration Course • Home Intravenous Therapy Program (HITP) – RN’s only 2.2 A prescriber order is required to start an IV except in an emergency situation or for restarts. -

Intravenous Fluids
See also IV fluid guidelines at www.rch.org.au/clinicalguide/cpg.cfm?doc_id=5203, contact email: [email protected] or [email protected] Intravenous fluids Hourly maintenance intravenous fluid requirements [Resuscitation bolus (Normal saline): 10-20 ml/kg] Weight (kg) 4 6 8 10 12 14 16 20 30 40 50 60 70 ml/hr 16 24 32 40 44 48 52 60 70 80 90 100 100 Reminders 1. Check weight of patient 2. Check maintenance fluids infusion rate according to weight. Remember that the maintenance fluid volume will need to be reduced in many unwell children, especially children with meningitis, bronchiolitis, pneumonia, some surgical problems and many children with hyponatraemia. 3. Consider why the patient needs IV fluid. If a child is prescribed anything other than a 0.9% or 0.45% NaCl solution, ask why. 4. Check the written order 5. If in doubt about fluids please ask, call a paediatric registrar, a consultant or the ICU Type of fluid Comment Na+ Cl- K+ Lactate Ca++ Glucose See also Clinical Practice Guidelines Intravenous Fluids (mmol/L) (mmol/L) (mmol/L) (mmol/L) (mmol/L) (gram/L) Monitor 0.9% NaCl Isotonic, contains no glucose 150 150 - - - - Clinical signs of (Normal Saline) Use for initial volume resuscitation, e.g. dehydration and over- septic shock, trauma. hydration 0.9% NaCl with 5% dextrose Use as maintenance fluid for suspected 150 150 50 (Normal saline with glucose) meningitis, acute neurological conditions, Weigh prior to where IV fluids are used for gastroenteritis or commencement of IV when the serum sodium is low. -

Clinical Pharmacology of Infusion Fluids
Clinical pharmacology of infusion fluids Robert G. Hahn Linköping University Post Print N.B.: When citing this work, cite the original article. Original Publication: Robert G. Hahn , Clinical pharmacology of infusion fluids, 2012, Acta Medica Lituanica, (19), 3. Licencee: Lithuanian Academy of Sciences http://www.lmaleidykla.lt/ojs/index.php/actamedicalituanica/index Postprint available at: Linköping University Electronic Press http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-91319 ACTA MEDICA LITUANICA. 2012. Vol. 19. No. 3. P. 210–212 © Lietuvos mokslų akademija, 2012 Clinical pharmacology of infusion fluids Robert G. Hahn Fluids are used for intravenous infusion during practically all surgeries, but several different compositions are available on the market. Södertälje Hospital, Crystalloid fluids comprise lactated or acetated Ringer solutions, nor- Södertälje, Sweden; mal saline, Plasma-Lyte, hypertonic saline, and glucose. They lack allergic Anaesthesia and properties but are prone to cause peripheral tissue oedema. Their turn- Intensive Care, over is governed by physiological factors such as dehydration and drug Linköping University, effects. Sweden Colloid fluids include hydroxyethyl starch, albumin, dextran, and gela- tin. These fluids have various degrees of allergic properties and do not promote peripheral oedema. Their half-life is usually about hours. Factors increasing the turnover rate are poorly known but might include inflam- matory states. Current debates include the widespread use of normal saline, which should be replaced by Ringer’s or Plasma-Lyte in most situations, and the kidney damage associated with the use of starch in septic patients. New studies show that hypertonic saline does not improve survival or neuro- logical damage in prehospital care. -

High Volume Sinonasal Budesonide Irrigations for Chronic Rhinosinusitis
oepidem ac io m lo Rudmik, Adv Pharmacoepidemiol Drug Saf 2014, 3:2 r g a y h & P Advances in Pharmacoepidemiology & DOI: 10.4172/2167-1052.1000148 D n i r u s g e c ISSN: 2167-1052 S n a a f v e t d y A Drug Safety Review Article Open Access High Volume Sinonasal Budesonide Irrigations for Chronic Rhinosinusitis: An Update on the Safety and Effectiveness Luke Rudmik* Division of Otolaryngology–Head and Neck Surgery, Department of Surgery, University of Calgary, Calgary, Alberta, Canada Abstract Chronic rhinosinusitis (CRS) is a common inflammatory disease of the paranasal sinuses associated with severe impairments in patient quality of life, sleep, and productivity. Topical corticosteroid therapy is a key component to a successful management plan for patients with CRS. Delivering topical medical therapies using high-volume sinonasal irrigations are commonly used following endoscopic sinus surgery (ESS) due to its proven efficacy for improving drug delivery into the paranasal sinuses. Topical high volume budesonide irrigations have become a popular off- label management strategy for CRS with the purpose to improve topical steroid delivery into the sinonasal cavities. Early evidence outlined in this review suggests that high volume sinonasal budesonide irrigations are an effective treatment modality in patients with CRS following ESS. Overall it appears that short-term use of this therapy is likely safe, however, future studies will need to assess the safety of higher doses and longer-term therapy of budesonide irrigations in patients with CRS. Keywords: Chronic rhinosinusitis; Sinusitis; Sinonasal; Nasal; mixing an active topical medical agent with an isotonic saline solution Budesonide; Topical Steroid; Safety; Irrigations; Corticosteroid; followed by a low-pressure delivery into the nasal cavity using either a Effectiveness squeeze bottle or neti pot. -

Maintenance Fluid Therapy with Saline, Dextrose-Supplemented Saline Or Lactated Ringer in Childhood: Short-Term Metabolic Effect
nutrients Article Maintenance Fluid Therapy with Saline, Dextrose-Supplemented Saline or Lactated Ringer in Childhood: Short-Term Metabolic Effects 1, 1,2,3, , 1 1 Alessandra Ricciuti y, Gregorio P. Milani * y , Silvia Tarantino , Roberta Ghilardi , Sebastiano A.G. Lava 4, Marco Alberzoni 2, Mario G. Bianchetti 5 and Carlo Agostoni 1,2,3 1 Pediatric Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy; [email protected] (A.R.); [email protected] (S.T.); [email protected] (R.G.); [email protected] (C.A.) 2 Department of Clinical Sciences and Community Health, Università degli Studi di Milano, 20122 Milan, Italy; [email protected] 3 Italian Society for Pediatric Gastroenterology Hepatology and Nutrition (SIGENP), 20126 Milan, Italy 4 Pediatric Cardiology Unit, Department of Pediatrics, Centre Hospitalier Universitaire Vaudois, and University of Lausanne, 1010 Lausanne, Switzerland; [email protected] 5 Università della Svizzera Italiana, 6900 Lugano, Switzerland; [email protected] * Correspondence: [email protected]; Tel.: +39-025-503-2266 These two authors equally contributed to the study. y Received: 29 March 2020; Accepted: 14 May 2020; Published: 17 May 2020 Abstract: Maintenance with isotonic fluids is recommended in children with gastroenteritis and failure of oral rehydration therapy. However, little is known on the short-term effects of the commonly prescribed intravenous solutions on metabolic balance in children. The aim of this study is to report on our experience with normal saline, dextrose-supplemented saline and lactated Ringer solution. Methods: A retrospective analysis from the charts of all previously apparently healthy children with acute gastroenteritis, mild to moderate dehydration and failure of oral rehydration, evaluated between January 2016 and December 2019 at our institution, was performed. -
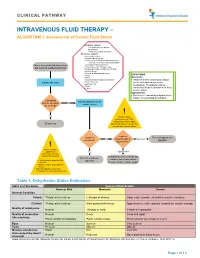
Intravenous Fluid Therapy – Algorithm 1
CLINICAL PATHWAY INTRAVENOUS FLUID THERAPY – ALGORITHM 1. Assessment of Overall Fluid Status Inclusion criteria: • All inpatients except those listed below • Patients pending admission Exclusion criteria: •Acute kidney injury •Chronic kidney disease •Endocrine or renal abnormalities leading to electrolyte derangements including DKA Patient who meets inclusion criteria •Oncology treatment protocol •Patients less than 30 days of age, and warrants supplemental fluids Including premature infants corrected for gestational age •Increased intracranial pressure •PICU DEFINITIONS •NICU Euvolemic: •Total Parenteral Nutrition dependent • Patient is at their ideal volume status Obtain vital signs •Pyloric Stenosis (neither dehydrated nor volume •Burn patients overloaded). The patient requires •Shock •Codes intravenous fluids to maintain their ideal volume status. Hypovolemic: • Patient is at least mildly dehydrated (see Table 1 for estimating dehydration) Can patient Assess patient’s current tolerate adequate No volume status enteral fluids? ! Yes Volume status assessment is 100% clinical. Do not rely upon Off pathway laboratory values to determine the patient’s volume status. Is patient Is patient Refer to hypovolemic No hypervolemic or Hypovolemic euvolemic? algorithm hypovolemic? ! Prior to starting a patient on Yes Hypervolemic maintenance IV fluids, consider the following: Reassess need for IV fluids and Refer to euvolemic consider issues with oncotic • Risk factors for abnormal ADH algorithm secretion pressure and/or cardiac output • Initial -
IDF Guide for Nurses Imimmmunnoogglolboubliun Ltinhe Trahpeyr Faopr Y Fopr Rpirmimaarryy I Mimmumnoudneoficdieenfciyc Ideisnecasy Es Diseases IDF GUIDE for NURSES
IDF Guide for Nurses ImImmmunnoogglolboubliun lTinhe Trahpeyr faopr y foPr rPirmimaarryy I mImmumnoudneoficdieenfciyc iDeisnecasy es Diseases IDF GUIDE FOR NURSES IMMUNOGLOBULIN THERAPY FOR PRIMARY IMMUNODEFICIENCY DISEASES THIRD EDITION COPYRIGHTS 2004, 2007, 2012 IMMUNE DEFICIENCY FOUNDATION Copyright 2012 by the Immune Deficiency Foundation, USA. PRINT: 12/2013 Readers may redistribute this publication to other individuals for non-commercial use, provided that the text, html codes, and this notice remain intact and unaltered in any way. The IDF Guide for Nurses may not be resold, reprinted or redistributed for compensation of any kind without prior written permission from the Immune Deficiency Foundation. If you have any questions about permission, please contact: Immune Deficiency Foundation, 40 West Chesapeake Avenue, Suite 308, Towson, MD 21204, USA, or by telephone: 800.296.4433. This publication has been made possible through a generous grant from IDF Guide for Nurses Immunoglobulin Therapy for Primary Immunodeficiency Diseases Third Edition Immune Deficiency Foundation 40 West Chesapeake Avenue, Suite 308 Towson, MD 21204 800.296.4433 www.primaryimmune.org Editor: M. Elizabeth M. Younger CRNP, PhD Johns Hopkins, Baltimore, Maryland Vice Chair, Immune Deficiency Foundation Nurse Advisory Committee Associate Editors: Rebecca H. Buckley, MD Duke University School of Medicine, Durham, NC Chair, Immune Deficiency Foundation Medical Advisory Committee Christine M. Belser Immune Deficiency Foundation, Towson, Maryland Kara Moran Immune Deficiency -

Two Doses of Early Intravenous Dexamethasone for the Prevention of Bronchopulmonary Dysplasia in Babies with Respiratory Distress Syndrome1
0031-3998/9413601-0122$03.00/0 PEDIATRIC RESEARCH Vol. 36, No. 1, 1994 Copyright 0 1994 International Pediatric Research Foundation, Inc. Printed in (I.S.A. Two Doses of Early Intravenous Dexamethasone for the Prevention of Bronchopulmonary Dysplasia in Babies with Respiratory Distress syndrome1 RAYMOND J. SANDERS,' CHRISTOPHER COX, DALE L. PHELPS, AND ROBERT A. SINKIN Departments of Pediatrics (Neonatology)[R. J. S., D. L. P., R.A. S. 1 and Biostatistics [C.C. I, Strong Children S Medical Center, Rochester, New York 14642 Bronchopulmonary dysplasia is an important complica- methasone group was 89% versus 67% in the placebo group tion of ventilation in babies for which treatment with ste- @ = 0.08, XZ analysis). Survival without bronchopulmo- roids has been advocated. We report the results of a phase nary dysplasia, diagnosed at 36 wk corrected gestational I study of early i.v. dexamethasone to prevent the devel- age, was 68% in the dexamethasone group versus 43% in opment of bronchopulmonary dysplasia in a high-risk pop- the placebo group @ = 0.14). Mean blood pressure was ulation of ventilated premature babies, < 30 wk gestation, elevated on study d 4 through 7 in the dexamethasone with surfactant-treated respiratory distress syndrome. This group, but this difference resolved by study d 10 without study used a limited dexamethasone dosing regimen to pharmacologic intervention. No differences in hyperglyce- minimize toxicity but used administration early in the mia, incidence of intraventricular hemorrhage (or its sever- course of acute lung disease to interrupt the injury cycle. ity), or days to regain birth weight were seen. -

Saline Solution
Preparing Saline Solution What will I need to prepare saline solution? Table salt (Sodium Chloride) Measuring Teaspoon Clean jar or other container with a lid. Reuse this container. What is the recipe for saline solution? 2 Teaspoons of table salt 1000 mL (4 cups) of warm tap water How do I prepare saline solution? Wash your hands well and rinse them with warm water. Pour 1000 mL (4 cups) of warm water Add 2 teaspoons of table salt into your container. Measure exact amount of salt to make sure it is correct. Mix until salt is completely dissolved. Label your container. *** Cut along dotted lines and tape onto your container*** _______________________________________________________________________ Normal Saline Recipe 2 Teaspoons of table salt 1000 mL (4 cups) of warm tap water -------------------------------------------------------------------------------------------------------------- Pediatric Surgery, C. S. Mott Children’s Hospital (734) 764 - 4151 - 1 - If you use well water, boil it for ten minutes, let cool for one hour, and then boil another ten minutes before mixing with salt. Normal saline solution can be stored at room temperature for three days in a closed container. What is the contact information? If you have any question, problems or concerns call the Pediatric Surgery clinic from 8-5:00pm Monday thru Friday, 734-764-4151. After 5:00pm or on the weekends if you have urgent issues call hospital paging at 734 936-4000 and ask the operator to page the Pediatric Surgeon “on call”. Disclaimer: This document contains information and/or instructional materials developed by the University of Michigan Health System (UMHS) for the typical patient with your condition. -

Crystalloids and Colloids Intravenous Therapy
CRYSTALLOIDS AND COLLOIDS INTRAVENOUS THERAPY NOAH CARPENTER, MD Dr. Noah Carpenter has practiced as a thoracic and peripheral vascular surgeon. He completed the Bachelor of Science in chemistry and medical school and training at the University of Manitoba. Dr. Carpenter completed surgical residency and fellowship at the University of Edmonton and Affiliated Hospitals in Edmonton, Alberta, and an additional Adult Cardiovascular and Thoracic Surgery fellowship at the University of Edinburgh, Scotland. He has specialized in microsurgical techniques, vascular endoscopy, laser and laparoscopic surgery in Brandon, Manitoba and Vancouver Island, British Columbia, Canada and in Colorado, Texas, and California. Dr. Carpenter has an Honorary Doctorate of Law from the University of Calgary, and was appointed a Citizen Ambassador to China, and has served as a member of the Native Physicians Association of Canada, the Canadian College of Health Service Executives, the Science Institute of the Northwest Territories, the Canada Science Council, and the International Society of Endovascular Surgeons, among others. He has been an inspiration to youth, motivating them to understand the importance of achieving higher education. Abstract A majority of hospital patients receive some form of intravenous therapy during a hospital stay, and a significant number of those patients require fluid replacement in the form of volume expanders. There are two types of volume expanders, crystalloids and colloids, and each has advantages and disadvantages. While there continues some controversy in the research over which volume expander is the best choice during specific situations of resuscitation, general medical wisdom exists that allows health clinicians to understand the best use of each and to provide patients with the most effective treatment possible. -

Instructions After Septoplasty And/Or Turbinate Reduction
INSTRUCTIONS AFTER SEPTOPLASTY AND/OR TURBINATE REDUCTION 1. Diet Patients may resume a regular diet without special restrictions. Many patients note a scratchy throat from the endotracheal tube for the first day and may prefer soft food initially. 2. Activity Light to moderate activity is recommended. Avoid heavy lifting and straining for the first week after surgery. Nose blowing initially should be gentle and minimal. Forceful nose blowing can lead to nasal bleeding. Elevating the head while resting or sleeping may be helpful. 3. Packing Your surgeon may place packing and/or plastic splints within your nasal cavity after the surgery. Your surgeon will let you know when these will be removed. 4. Nasal Saline Irrigation Your physician may recommend mechanically cleaning your nose and sinuses with nasal saline. This can remove blood and mucus from the nose and help with healing after the surgery. Nasal saline spray is available over the counter. You can also make a saline solution with the following recipe: -1/2 tsp table salt -12 ounces of water (clean tap or boiled water) -Pinch of baking soda 5. Pain Some pain after nasal surgery is expected. It is generally of moderate severity. Take the pain medicine as prescribed by your physician. 6. Fever Low-grade temperature after nasal surgery is common. You should notify our office if you temperature is 101ºF or greater. 7. Bleeding Some bloody nasal and postnasal drainage after nasal surgery is expected. You should expect light bloody drainage for the first 1-2 days. Call the office if your bleeding becomes excessive. -

0.18% Saline Or 4% Glucose IV Solution Safety Risks In
1 MHRA UK PUBLIC ASSESSMENT REPORT Intravenous 0.18% saline/4% glucose solution (‘hypotonic saline’): do not use in children except in specialist settings under expert supervision October 2012 Plain-language summary 2 1. Introduction 4 2. Background 4 3. Results 9 4. Discussion 17 5. New advice 17 6. References 19 7. Glossary 21 2 PLAIN-LANGUAGE SUMMARY KEY MESSAGE: Intravenous hypotonic saline (0.18% saline/4% glucose solution) can cause dangerously low levels of sodium in children, which in some cases has lead to swelling of the brain and death. Therefore, 0.18% saline/4% glucose solution must not be used in children aged 16 years or less, unless it is in a specialist setting, such as kidney, heart, liver, high- dependency, or intensive care units, and there is expert medical supervision. The child must be carefully monitored throughout treatment. Background The Medicines and Healthcare products Regulatory Agency (MHRA) is the UK government agency responsible for regulating medicines and medical devices. We continually review the safety of medicines and vaccines in the UK1, and inform healthcare professionals and the public of the latest updates through several means, including public assessment reports. This report discusses a medical solution used in hospitals called hypotonic saline (0.18% saline/4% glucose), and its risks and benefits when used in children. Hypotonic saline is given to patients intravenously2 by health professionals in a hospital to maintain or replace their fluid and salt requirements. It has been used for this purpose for since the 1950s in both adults and children. There are several solutions of different compositions available in hospitals for intravenous fluid replacement therapy.