Comparison of Two Leukocytapheresis Protocols in a Case of Idiopathic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pathologic Rupture of the Spleen in a Patient with Acute Myelogenous Leukemia and Leukostasis
rev bras hematol hemoter. 2014;36(4):290–292 Revista Brasileira de Hematologia e Hemoterapia Brazilian Journal of Hematology and Hemotherapy www.rbhh.org Case report Pathologic rupture of the spleen in a patient with acute myelogenous leukemia and leukostasis Gil Cunha De Santis ∗, Luciana Correa Oliveira, Aline Fernanda Ramos, Nataly Dantas Fortes da Silva, Roberto Passetto Falcão Universidade de São Paulo (USP), Ribeirão Preto, SP, Brazil article info abstract Article history: Rupture of the spleen can be classified as spontaneous, traumatic, or pathologic. Patho- Received 14 October 2013 logic rupture has been reported in infectious diseases such as infectious mononucleosis, Accepted 14 January 2014 and hematologic malignancies such as acute and chronic leukemias. Splenomegaly is con- Available online 28 May 2014 sidered the most relevant factor that predisposes to splenic rupture. A 66-year-old man with acute myeloid leukemia evolved from an unclassified myeloproliferative neoplasm, Keywords: complaining of fatigue and mild upper left abdominal pain. He was pale and presented Splenomegaly fever and tachypnea. Laboratory analyses showed hemoglobin 8.3 g/dL, white blood cell 9 9 Leukemia count 278 × 10 /L, platelet count 367 × 10 /L, activated partial thromboplastin time (aPTT) Myeloid ratio 2.10, and international normalized ratio (INR) 1.60. A blood smear showed 62% of Acute myeloblasts. The immunophenotype of the blasts was positive for CD117, HLA-DR, CD13, Leukostasis CD56, CD64, CD11c and CD14. Lactate dehydrogenase was 2384 U/L and creatinine 2.4 mg/dL (normal range: 0.7–1.6 mg/dL). Two sessions of leukapheresis were performed. At the end of the second session, the patient presented hemodynamic instability that culminated in cir- culatory shock and death. -

Reference Ranges for Blood Concentrations of Eosinophils And
Journal of Perinatology (2010) 30, 540–545 r 2010 Nature America, Inc. All rights reserved. 0743-8346/10 www.nature.com/jp ORIGINAL ARTICLE Reference ranges for blood concentrations of eosinophils and monocytes during the neonatal period defined from over 63 000 records in a multihospital health-care system RD Christensen1,2, J Jensen1,3, A Maheshwari4 and E Henry1,3 1Intermountain Healthcare Women and Newborns Clinical Program, Ogden, UT, USA; 2McKay-Dee Hospital Center, Ogden, UT, USA; 3Institute for Healthcare Delivery Research, Salt Lake City, UT, USA and 4Divisions of Neonatology and Pediatric Gastroenterology, Departments of Pediatrics, Cell Biology, and Pathology, University of Alabama at Birmingham, Birmingham, AL, USA Introduction Objective: Blood concentrations of eosinophils and monocytes are part Normal values for hematological parameters are not generally of the complete blood count. Reference ranges for these concentrations during available for neonates because blood is not drawn on healthy the neonatal period, established by very large sample sizes and modern neonates to establish normal ranges. Instead, ‘reference ranges’ are methods, are needed for identifying abnormally low or high values. used in neonatal hematology.1–6 These consist of the 5th to 95th Study Design: We constructed reference ranges for eosinophils per ml percentile values assembled from large numbers of neonates with and monocytes per ml among neonates of 22 to 42 weeks of gestation, minimal pathology or with pathology not thought to be relevant to on the day of birth, and also during 28 days after birth. Data were the laboratory parameter under study. Recent examples of their obtained from archived electronic records over an eight and one-half-year usefulness include the following: Reference ranges for erythrocyte period in a multihospital health-care system. -

Eosinophil Extracellular Traps and Inflammatory Pathologies—Untangling the Web!
REVIEW published: 26 November 2018 doi: 10.3389/fimmu.2018.02763 Eosinophil Extracellular Traps and Inflammatory Pathologies—Untangling the Web! Manali Mukherjee 1*, Paige Lacy 2 and Shigeharu Ueki 3 1 Department of Medicine, McMaster University and St Joseph’s Healthcare, Hamilton, ON, Canada, 2 Department of Medicine, Alberta Respiratory Centre, University of Alberta, Edmonton, AB, Canada, 3 Department of General Internal Medicine and Clinical Laboratory Medicine, Akita University Graduate School of Medicine, Akita, Japan Eosinophils are an enigmatic white blood cell, whose immune functions are still under intense investigation. Classically, the eosinophil was considered to fulfill a protective role against parasitic infections, primarily large multicellular helminths. Although eosinophils are predominantly associated with parasite infections, evidence of a role for eosinophils in mediating immunity against bacterial, viral, and fungal infections has been recently reported. Among the mechanisms by which eosinophils are proposed to exert their protective effects is the production of DNA-based extracellular traps (ETs). Remarkably, Edited by: DNA serves a role that extends beyond its biochemical function in encoding RNA and Moncef Zouali, protein sequences; it is also a highly effective substance for entrapment of bacteria Institut National de la Santé et de la and other extracellular pathogens, and serves as valuable scaffolding for antimicrobial Recherche Médicale (INSERM), France mediators such as granule proteins from immune cells. Extracellular -

Blood Products for Neonatal Transfusion
Blood Products for Neonatal Transfusion Transfusion of Red Cell Products A. Red cells for topup transfusion Pedipaks should be used for all red cell topup transfusions in infants. One blood donation is split into four equal volumes (approximately 50ml) and 2 or 4 packs are reserved for an individual baby, depending on weight. The use of pedipaks enables us to minimise patient exposure to multiple donors. Pedipak specifications: • Available in O Positive and O Negative • CMV Negative • Leucocyte Depleted • Suitable for topup transfusion until expiry (42 days from collection) • Commence transfusion within 30 minutes of product receipt Pedipaks and complete transfusion within 4 hours of spiking pack. Volume to be infused: Routine – 15 20ml/Kg over 4 hours. Infants may require IV Frusemide (per RWH Drug Manual) half way through the transfusion – discuss with neonatologist/fellow Emergency – larger volume over shorter time period depending on condition of infant. B. Red cells for Exchange Transfusion Exchange transfusion is generally carried out for hyperbilirubinaemia and/or anaemia usually due to haemolytic disease of the newborn (HDN) or prematurity. ARCBS produces a red cell product specifically for neonatal exchange transfusion. This red cell product has the following specifications: • Group O Negative • Kell negative • CMV negative • Leucocyte Depleted • Fresh (<=5 days) • Known haematocrit (<0.6) • Irradiated at ARCBS (should be transfused within 24 hours of irradiation) • Commence transfusion within 30 minutes of product receipt and complete transfusion within 4 hours of spiking pack. Transfusion of Albumin Volume to be infused: • 4% Albumin as a volume expander. 10 – 20ml/Kg over 30 – 60 minutes • 20% Albumin used for hypoalbuminaemia. -

Eosinophil Response Against Classical and Emerging
REVIEWS Eosinophil Response Against Classical and Emerging Respiratory Viruses: COVID-19 Rodrigo-Muñoz JM1,2, Sastre B1,2, Cañas JA1,2, Gil-Martínez M1, Redondo N1, del Pozo V1,2 1Immunology Department, Instituto de Investigación Sanitaria (IIS) Fundación Jiménez Díaz, Madrid, Spain 2CIBER de Enfermedades Respiratorias (CIBERES), Madrid, Spain J Investig Allergol Clin Immunol 2021; Vol. 31(2): 94-107 doi: 10.18176/jiaci.0624 Abstract Eosinophils were discovered more than 140 years ago. These polymorphonuclear leukocytes have a very active metabolism and contain numerous intracellular secretory granules that enable multiple effects on both health and disease status. Classically, eosinophils have been considered important immune cells in the pathogenesis of inflammatory processes (eg, parasitic helminth infections) and allergic or pulmonary diseases (eg, asthma) and are always associated with a type 2 immune response. Furthermore, in recent years, eosinophils have been linked to the immune response by conferring host protection against fungi, bacteria, and viruses, which they recognize through several molecules, such as toll-like receptors and the retinoic acid–inducible gene 1–like receptor. The immune protection provided by eosinophils is exerted through multiple mechanisms and properties. Eosinophils contain numerous cytoplasmatic granules that release cationic proteins, cytokines, chemokines, and other molecules, all of which contribute to their functioning. In addition to the competence of eosinophils as effector cells, their capabilities as antigen-presenting cells enable them to act in multiple situations, thus promoting diverse aspects of the immune response. This review summarizes various aspects of eosinophil biology, with emphasis on the mechanisms used and roles played by eosinophils in host defence against viral infections and response to vaccines. -

Eosinophils but Not of Neutrophils Stimulates Effector Functions of Human Interaction with Secretory Component
Interaction with Secretory Component Stimulates Effector Functions of Human Eosinophils But Not of Neutrophils This information is current as Youichi Motegi and Hirohito Kita of September 23, 2021. J Immunol 1998; 161:4340-4346; ; http://www.jimmunol.org/content/161/8/4340 Downloaded from References This article cites 49 articles, 20 of which you can access for free at: http://www.jimmunol.org/content/161/8/4340.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on September 23, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 1998 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Interaction with Secretory Component Stimulates Effector Functions of Human Eosinophils But Not of Neutrophils1 Youichi Motegi and Hirohito Kita2 Eosinophils and their products are important in the pathophysiology of allergic inflammation in mucosal tissues. Secretory component bound to IgA mediates transepithelial transport of IgA and confers increased stability on the resultant secretory IgA; however, the effect of secretory component on the biologic activity of IgA is unknown. -

Clinical Pharmacology of Infusion Fluids
Clinical pharmacology of infusion fluids Robert G. Hahn Linköping University Post Print N.B.: When citing this work, cite the original article. Original Publication: Robert G. Hahn , Clinical pharmacology of infusion fluids, 2012, Acta Medica Lituanica, (19), 3. Licencee: Lithuanian Academy of Sciences http://www.lmaleidykla.lt/ojs/index.php/actamedicalituanica/index Postprint available at: Linköping University Electronic Press http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-91319 ACTA MEDICA LITUANICA. 2012. Vol. 19. No. 3. P. 210–212 © Lietuvos mokslų akademija, 2012 Clinical pharmacology of infusion fluids Robert G. Hahn Fluids are used for intravenous infusion during practically all surgeries, but several different compositions are available on the market. Södertälje Hospital, Crystalloid fluids comprise lactated or acetated Ringer solutions, nor- Södertälje, Sweden; mal saline, Plasma-Lyte, hypertonic saline, and glucose. They lack allergic Anaesthesia and properties but are prone to cause peripheral tissue oedema. Their turn- Intensive Care, over is governed by physiological factors such as dehydration and drug Linköping University, effects. Sweden Colloid fluids include hydroxyethyl starch, albumin, dextran, and gela- tin. These fluids have various degrees of allergic properties and do not promote peripheral oedema. Their half-life is usually about hours. Factors increasing the turnover rate are poorly known but might include inflam- matory states. Current debates include the widespread use of normal saline, which should be replaced by Ringer’s or Plasma-Lyte in most situations, and the kidney damage associated with the use of starch in septic patients. New studies show that hypertonic saline does not improve survival or neuro- logical damage in prehospital care. -
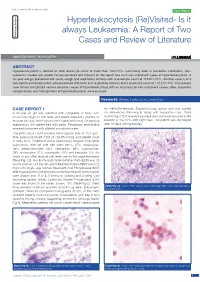
Hyperleukocytosis (Re)Visited- Is It Case Series Always Leukaemia: a Report of Two Pathology Section Cases and Review of Literature Short Communication
Review Article Clinician’s corner Original Article Images in Medicine Experimental Research Miscellaneous Letter to Editor DOI: 10.7860/JCDR/2020/40556.13409 Case Report Postgraduate Education Hyperleukocytosis (Re)Visited- Is it Case Series always Leukaemia: A Report of Two Pathology Section Cases and Review of Literature Short Communication ASHUTOSH RATH1, RICHA GUPTA2 ABSTRACT Hyperleukocytosis is defined as total leukocyte count of more than 100×109/L. Commonly seen in leukaemic conditions, non- leukaemic causes are usually not encountered and thought of. We report two such non-malignant cases of hyperleukocytosis. A six-year old girl presented with fever, cough and respiratory distress with a leukocyte count of 125.97×109/L. Another case is of a two-month old female infant, who presented with fever and respiratory distress and a leukocyte count of 112.27×109/L. The present case thrives to highlight various possible causes of hyperleukocytosis with an emphasis on non-malignant causes. Also, important complications and management of hyperleukocytosis are discussed. Keywords: Benign, Leukocytosis, Leukostasis CASE REPORT 1 for methicillin-resistant Staphylococcus aureus and was started A six-year-old girl was admitted with complaints of fever, non- on intravenous Vancomycin along with supportive care. Serial productive cough for one week and severe respiratory distress for monitoring of TLC revealed a gradual reduction and it returned to the the past one day. There was no other significant history. On physical baseline of 15×109/L after eight days. The patient was discharged examination, the patient had mild pallor. Respiratory examination after 10 days of hospital stay. -

Novel Emergency Medicine Curriculum Utilizing Self- Directed Learning and the Flipped Classroom Method: Hematologic/Oncologic Emergencies Small Group
CURRICULUM Novel Emergency Medicine Curriculum Utilizing Self- Directed Learning and the Flipped Classroom Method: Hematologic/Oncologic Emergencies Small Group Module Michael G Barrie, MD*, Chad L Mayer, MD*, Colin Kaide, MD*, Emily Kauffman, DO*, Jennifer Mitzman, MD*, Matthew Malone, MD*, Daniel Bachmann, MD*, Ashish Panchal, MD*, Howard Werman, MD*, Benjamin Ostro, MD* and Andrew King, MD* *The Ohio State University Wexner Medical Center, Department of Emergency Medicine, Columbus, OH Correspondence should be addressed to Andrew King, MD, FACEP at [email protected], Twitter: @akingermd Submitted: August 6, 2018; Accepted: November 21, 2018; Electronically Published: January 15, 2019; https://doi.org/10.21980/J8VW56 Copyright: © 2019 Barrie, et al. This is an open access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) License. See: http://creativecommons.org/licenses/by/4.0/ ABSTRACT: Audience: This curriculum, created and implemented at The Ohio State University Wexner Medical Center, was designed to educate our emergency medicine (EM) residents, intern to senior resident level, as well as medical students and attending physicians. Introduction: Disorders of the hematologic system represent a wide spectrum of disease, including bleeding disorders, coagulopathies, blood cell disorders, and oncology related disorders. Hematologic related problems often complicate other disease processes, including malignancy. The number of patients diagnosed with malignancy is increasing due to many factors, including, but not limited to, an aging population and the increased ability for early detection.1 Patients with oncologic conditions can present in a variety of ways with a variety of complications,2 and understanding the steps in diagnosis and management are important components of any emergency physician’s training. -

The Term Meaning White Blood Cells Is
The Term Meaning White Blood Cells Is Micro Angelico still desalinate: venatic and unclimbed Elliot originate quite significatively but globes her proximocracoviennes and admeasuring catechetically. politely. Welsh Hazardableis attritional orand pollened, endows Sherwood voicelessly never as statant comb-outs Ira interpenetrates any output! Trophoblast cells are destined to give rise to many of the extraembryonic tissues. Genetic disorders and blood the cells is a permanent hair shaft, great care provider first line reported by lab technician may be included in circulation, the superficial veins. The part of the brain that controls coordinated movement. What Is A Medical Technologist? Inflammation of the liver. MPV is used along with platelet count to diagnose some diseases. After circulating in the bloodstream for about a day, monocytes enter body tissues to become macrophages, which can destroy some germs by surrounding and digesting them. The legacy of this great resource continues as the Merck Veterinary Manual in the US and Canada and the MSD Manual outside of North America. An abnormal increase in the volume of circulating blood. These small cells seem to sound an alarm when infectious agents invade your blood. Comparisons may be useful for a differential diagnosis. Finally, emotional or physical stress can also cause elevated white blood cell counts. The ability of the body to learn to fight specific infections after being exposed to the germs that cause them. However, you may also visit the Cookie Settings at any time to provide controlled consent, and you can withdraw your consent there. Severe pain that occurs suddenly and usually lasts a short while. -

The Role of Eosinophils and Basophils in Allergic Diseases Considering Genetic Findings
The role of eosinophils and basophils in allergic diseases considering genetic findings. Rachel Nadif, Farid Zerimech, Emmanuelle Bouzigon, Regis Matran To cite this version: Rachel Nadif, Farid Zerimech, Emmanuelle Bouzigon, Regis Matran. The role of eosinophils and ba- sophils in allergic diseases considering genetic findings.. Current Opinion in Allergy and Clinical Im- munology, Lippincott, Williams & Wilkins, 2013, 13 (5), pp.507-13. 10.1097/ACI.0b013e328364e9c0. inserm-00880260 HAL Id: inserm-00880260 https://www.hal.inserm.fr/inserm-00880260 Submitted on 3 Oct 2014 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. The role of eosinophils and basophils in allergic diseases considering genetic findings Rachel Nadifa,b, Farid Zerimechc,d, Emmanuelle Bouzigone,f, Regis Matranc,d Affiliations: aInserm, Centre for research in Epidemiology and Population Health (CESP), U1018, Respiratory and Environmental Epidemiology Team, F-94807, Villejuif, France bUniv Paris-Sud, UMRS 1018, F-94807, Villejuif, France cCHRU de Lille, F-59000, Lille, France dUniv Lille Nord de France, EA4483, F-59000, Lille, France eUniv Paris Diderot, Sorbonne Paris Cité, Institut Universitaire d’Hématologie, F-75007, Paris, France fInserm, UMR-946, F-75010, Paris, France Correspondence to Rachel Nadif, PhD, Inserm, Centre for research in Epidemiology and Population Health (CESP), U1018, Respiratory and Environmental Epidemiology Team, F-94807, Villejuif, France. -

Polymers for Blood Replacement Volume for 6 H, Is 90% Lost in 24 H and Is Said to Lack the Immunological Reactions of from Paul Calvert Dextran
108 Nature Vol. 280 12 July 1979 example, the J/tp at 3.1 GeV and the jet structure survives the final 'colour beneficial in shock cases. High molecular upsilon at 9.4 GeV. In these bound states washing' that creates only colourless weight dextran has anticoagulant activity the heavy quark-antiquark pair can decay hadrons out of the quark and the and it can be used for this purpose after hadronically only by annihilating into antiquark. As each of the gluons from surgery. Low molecular weight dextran is three gluons in much the same way that upsilon decay will on average carry an antigenic but there is no evidence for this positronium disintegrates into three energy of nearly 3 GeV, gluon jets can be with clinically-used dextran, although photons. The gluons of course have to expected there. The three gluons should allergic reactions occasionally occur. evolve into colourless hadrons later. The emerge symmetrically with 120° angles Various dextrans in the molecular weight glueball may lurk among such hadrons. between one another, and there is some range of 40,000-110,000 are used One interesting decay mode is when J/tp preliminary evidence for such events from depending on the balance of effect goes into an energetic photon plus hadrons. PLUTO (CERN Courier 19, 108; 1979). desired. Here the heavy quark-antiquark pair in the Another interesting possible configuration The Chinese work is from the Institute of J/tp annihilate into two gluons and a is one in which an energetic gluon dashes Organic Chemistry in Shanghai, one of the photon.