Drinking Water Microflora Biofilms and Chlorine Susceptibility
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Which Organisms Are Used for Anti-Biofouling Studies
Table S1. Semi-systematic review raw data answering: Which organisms are used for anti-biofouling studies? Antifoulant Method Organism(s) Model Bacteria Type of Biofilm Source (Y if mentioned) Detection Method composite membranes E. coli ATCC25922 Y LIVE/DEAD baclight [1] stain S. aureus ATCC255923 composite membranes E. coli ATCC25922 Y colony counting [2] S. aureus RSKK 1009 graphene oxide Saccharomycetes colony counting [3] methyl p-hydroxybenzoate L. monocytogenes [4] potassium sorbate P. putida Y. enterocolitica A. hydrophila composite membranes E. coli Y FESEM [5] (unspecified/unique sample type) S. aureus (unspecified/unique sample type) K. pneumonia ATCC13883 P. aeruginosa BAA-1744 composite membranes E. coli Y SEM [6] (unspecified/unique sample type) S. aureus (unspecified/unique sample type) graphene oxide E. coli ATCC25922 Y colony counting [7] S. aureus ATCC9144 P. aeruginosa ATCCPAO1 composite membranes E. coli Y measuring flux [8] (unspecified/unique sample type) graphene oxide E. coli Y colony counting [9] (unspecified/unique SEM sample type) LIVE/DEAD baclight S. aureus stain (unspecified/unique sample type) modified membrane P. aeruginosa P60 Y DAPI [10] Bacillus sp. G-84 LIVE/DEAD baclight stain bacteriophages E. coli (K12) Y measuring flux [11] ATCC11303-B4 quorum quenching P. aeruginosa KCTC LIVE/DEAD baclight [12] 2513 stain modified membrane E. coli colony counting [13] (unspecified/unique colony counting sample type) measuring flux S. aureus (unspecified/unique sample type) modified membrane E. coli BW26437 Y measuring flux [14] graphene oxide Klebsiella colony counting [15] (unspecified/unique sample type) P. aeruginosa (unspecified/unique sample type) graphene oxide P. aeruginosa measuring flux [16] (unspecified/unique sample type) composite membranes E. -

Phage-Induced Lysis Enhances Biofilm Formation in Shewanella Oneidensis MR-1
The ISME Journal (2011) 5, 613–626 & 2011 International Society for Microbial Ecology All rights reserved 1751-7362/11 www.nature.com/ismej ORIGINAL ARTICLE Phage-induced lysis enhances biofilm formation in Shewanella oneidensis MR-1 Julia Go¨deke, Kristina Paul, Ju¨ rgen Lassak and Kai M Thormann Department of Ecophysiology, Max-Planck-Institut fu¨r Terrestrische Mikrobiologie, Marburg, Germany Shewanella oneidensis MR-1 is capable of forming highly structured surface-attached communities. By DNase I treatment, we demonstrated that extracellular DNA (eDNA) serves as a structural component in all stages of biofilm formation under static and hydrodynamic conditions. We determined whether eDNA is released through cell lysis mediated by the three prophages LambdaSo, MuSo1 and MuSo2 that are harbored in the genome of S. oneidensis MR-1. Mutant analyses and infection studies revealed that all three prophages may individually lead to cell lysis. However, only LambdaSo and MuSo2 form infectious phage particles. Phage release and cell lysis already occur during early stages of static incubation. A mutant devoid of the prophages was significantly less prone to lysis in pure culture. In addition, the phage-less mutant was severely impaired in biofilm formation through all stages of development, and three-dimensional growth occurred independently of eDNA as a structural component. Thus, we suggest that in S. oneidensis MR-1 prophage-mediated lysis results in the release of crucial biofilm-promoting factors, in particular eDNA. The ISME Journal (2011) 5, 613–626; doi:10.1038/ismej.2010.153; published online 21 October 2010 Subject Category: microbe–microbe and microbe–host interactions Keywords: Shewanella; biofilm; eDNA; lysis; phage Introduction been demonstrated to adhere to various surfaces and form biofilms (Bagge et al., 2001; Thormann et al., Shewanella oneidensis MR-1 belongs to the Gram- 2004, 2005, 2006; Teal et al., 2006; McLean et al., negative g-proteobacteria and is characterized by an 2008a; Zhang et al., 2010). -

Fish Bacterial Flora Identification Via Rapid Cellular Fatty Acid Analysis
Fish bacterial flora identification via rapid cellular fatty acid analysis Item Type Thesis Authors Morey, Amit Download date 09/10/2021 08:41:29 Link to Item http://hdl.handle.net/11122/4939 FISH BACTERIAL FLORA IDENTIFICATION VIA RAPID CELLULAR FATTY ACID ANALYSIS By Amit Morey /V RECOMMENDED: $ Advisory Committe/ Chair < r Head, Interdisciplinary iProgram in Seafood Science and Nutrition /-■ x ? APPROVED: Dean, SchooLof Fisheries and Ocfcan Sciences de3n of the Graduate School Date FISH BACTERIAL FLORA IDENTIFICATION VIA RAPID CELLULAR FATTY ACID ANALYSIS A THESIS Presented to the Faculty of the University of Alaska Fairbanks in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE By Amit Morey, M.F.Sc. Fairbanks, Alaska h r A Q t ■ ^% 0 /v AlA s ((0 August 2007 ^>c0^b Abstract Seafood quality can be assessed by determining the bacterial load and flora composition, although classical taxonomic methods are time-consuming and subjective to interpretation bias. A two-prong approach was used to assess a commercially available microbial identification system: confirmation of known cultures and fish spoilage experiments to isolate unknowns for identification. Bacterial isolates from the Fishery Industrial Technology Center Culture Collection (FITCCC) and the American Type Culture Collection (ATCC) were used to test the identification ability of the Sherlock Microbial Identification System (MIS). Twelve ATCC and 21 FITCCC strains were identified to species with the exception of Pseudomonas fluorescens and P. putida which could not be distinguished by cellular fatty acid analysis. The bacterial flora changes that occurred in iced Alaska pink salmon ( Oncorhynchus gorbuscha) were determined by the rapid method. -

Phenotypic and Genetic Diversity of Pseudomonads
PHENOTYPIC AND GENETIC DIVERSITY OF PSEUDOMONADS ASSOCIATED WITH THE ROOTS OF FIELD-GROWN CANOLA A Thesis Submitted to the College of Graduate Studies and Research In Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy In the Department of Applied Microbiology and Food Science University of Saskatchewan Saskatoon By Danielle Lynn Marie Hirkala © Copyright Danielle Lynn Marie Hirkala, November 2006. All rights reserved. PERMISSION TO USE In presenting this thesis in partial fulfilment of the requirements for a Postgraduate degree from the University of Saskatchewan, I agree that the Libraries of this University may make it freely available for inspection. I further agree that permission for copying of this thesis in any manner, in whole or in part, for scholarly purposes may be granted by the professor or professors who supervised my thesis work or, in their absence, by the Head of the Department or the Dean of the College in which my thesis work was done. It is understood that any copying or publication or use of this thesis or parts thereof for financial gain shall not be allowed without my written permission. It is also understood that due recognition shall be given to me and to the University of Saskatchewan in any scholarly use which may be made of any material in my thesis. Requests for permission to copy or to make other use of material in this thesis in whole or part should be addressed to: Head of the Department of Applied Microbiology and Food Science University of Saskatchewan Saskatoon, Saskatchewan, S7N 5A8 i ABSTRACT Pseudomonads, particularly the fluorescent pseudomonads, are common rhizosphere bacteria accounting for a significant portion of the culturable rhizosphere bacteria. -

Diaphorobacter Nitroreducens Gen. Nov., Sp. Nov., a Poly (3
J. Gen. Appl. Microbiol., 48, 299–308 (2002) Full Paper Diaphorobacter nitroreducens gen. nov., sp. nov., a poly(3-hydroxybutyrate)-degrading denitrifying bacterium isolated from activated sludge Shams Tabrez Khan and Akira Hiraishi* Department of Ecological Engineering, Toyohashi University of Technology, Toyohashi 441–8580, Japan (Received August 12, 2002; Accepted October 23, 2002) Three denitrifying strains of bacteria capable of degrading poly(3-hydroxybutyrate) (PHB) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) were isolated from activated sludge and characterized. All of the isolates had almost identical phenotypic characteristics. They were motile gram-negative rods with single polar flagella and grew well with simple organic com- pounds, as well as with PHB and PHBV, as carbon and energy sources under both aerobic and anaerobic denitrifying conditions. However, none of the sugars tested supported their growth. The cellular fatty acid profiles showed the presence of C16:1w7cis and C16:0 as the major com- ponents and of 3-OH-C10:0 as the sole component of hydroxy fatty acids. Ubiquinone-8 was de- tected as the major respiratory quinone. A 16S rDNA sequence-based phylogenetic analysis showed that all the isolates belonged to the family Comamonadaceae, a major group of b-Pro- teobacteria, but formed no monophyletic cluster with any previously known species of this fam- (DSM 13225؍) ily. The closest relative to our strains was an unidentified bacterium strain LW1 (99.9% similarity), reported previously as a 1-chloro-4-nitrobenzene degrading bacterium. DNA- DNA hybridization levels among the new isolates were more than 60%, whereas those between our isolates and strain DSM 13225 were less than 50%. -

Olabode Ope Samuel.Pdf (6.682Mb)
Faculty of Science and Technology MASTER’S THESIS Study program/ Specialization: Spring Semester, 2017 Master´s degree in Biological Chemistry Open/Restricted Writer: Ope Samuel Olabode ………………………………………………….. (Writer’s signature) Faculty Supervisor: Prof. Cathrine Lillo External Supervisor(s): Title of Thesis: The effect of plant growth-promoting bacteria on wild type, protein phosphatase 2A catalytic subunit mutants of Arabidopsis thaliana and Solanaceae lycopersicum (Gemini tomato) Credits (ECTS): 60 Key words: Arabidopsis Pages:54 thaliana, PP2A, RT-PCR, VOCs, ISR, + Enclosures:77 Stavanger, June 15, 2017 Date/Year Table of contents Table of contents .....................................................................................................................................i-iii Acknowledgement .................................................................................................................................... iv Abstracts ..................................................................................................................................................... v Abbreviation ............................................................................................................................................. vi List of Figures ................................................................................................................................... vii-viii List of Tables ............................................................................................................................................ -

Transfer of Several Phytopathogenic Pseudomonas Species to Acidovorax As Acidovorax Avenae Subsp
INTERNATIONALJOURNAL OF SYSTEMATICBACTERIOLOGY, Jan. 1992, p. 107-119 Vol. 42, No. 1 0020-7713/92/010107-13$02 .OO/O Copyright 0 1992, International Union of Microbiological Societies Transfer of Several Phytopathogenic Pseudomonas Species to Acidovorax as Acidovorax avenae subsp. avenae subsp. nov., comb. nov. , Acidovorax avenae subsp. citrulli, Acidovorax avenae subsp. cattleyae, and Acidovorax konjaci A. WILLEMS,? M. GOOR, S. THIELEMANS, M. GILLIS,” K. KERSTERS, AND J. DE LEY Laboratorium voor Microbiologie en microbiele Genetica, Rijksuniversiteit Gent, K.L. Ledeganckstraat 35, B-9000 Ghent, Belgium DNA-rRNA hybridizations, DNA-DNA hybridizations, polyacrylamide gel electrophoresis of whole-cell proteins, and a numerical analysis of carbon assimilation tests were carried out to determine the relationships among the phylogenetically misnamed phytopathogenic taxa Pseudomonas avenue, Pseudomonas rubrilineans, “Pseudomonas setariae, ” Pseudomonas cattleyae, Pseudomonas pseudoalcaligenes subsp. citrulli, and Pseudo- monas pseudoalcaligenes subsp. konjaci. These organisms are all members of the family Comamonadaceae, within which they constitute a separate rRNA branch. Only P. pseudoalcaligenes subsp. konjaci is situated on the lower part of this rRNA branch; all of the other taxa cluster very closely around the type strain of P. avenue. When they are compared phenotypically, all of the members of this rRNA branch can be differentiated from each other, and they are, as a group, most closely related to the genus Acidovorax. DNA-DNA hybridization experiments showed that these organisms constitute two genotypic groups. We propose that the generically misnamed phytopathogenic Pseudomonas species should be transferred to the genus Acidovorax as Acidovorax avenue and Acidovorax konjaci. Within Acidovorax avenue we distinguished the following three subspecies: Acidovorax avenue subsp. -

Activities and Prevalence of Proteobacteria Members Colonizing Echinacea Purpurea Fully Account for Macro- Phage Activation Exhibited by Extracts of This Botanical
1258 Original Papers Activities and Prevalence of Proteobacteria Members Colonizing Echinacea purpurea Fully Account for Macro- phage Activation Exhibited by Extracts of This Botanical Authors Mona H. Haron 1*, Heather L. Tyler 2, 3*, Nirmal D. Pugh 1, Rita M. Moraes1, Victor L. Maddox4, Colin R. Jackson 2, David S. Pasco1,5 Affiliations The affiliations are listed at the end of the article Key words Abstract highest potency for in vitro macrophage activa- l" Echinacea purpurea ! tion and were the most predominant taxa. Fur- l" Asteraceae Evidence supports the theory that bacterial com- thermore, the mean activity exhibited by the l" immunostimulatory munities colonizing Echinacea purpurea contrib- Echinacea extracts could be solely accounted for l" macrophage activation ute to the innate immune enhancing activity of by the activities and prevalence of Proteobacteria l" bacteria l" proteobacteria this botanical. Previously, we reported that only members comprising the plant-associated bacte- l" endophytes about half of the variation in in vitro monocyte rial community. The efficacy of E. purpurea mate- stimulating activity exhibited by E. purpurea ex- rial for use against respiratory infections may be tracts could be accounted for by total bacterial determined by the Proteobacterial community load within the plant material. In the current composition of this plant, since ingestion of bac- study, we test the hypothesis that the type of bac- teria (probiotics) is reported to have a protective teria, in addition to bacterial load, is necessary to effect against this health condition. fully account for extract activity. Bacterial com- munity composition within commercial and freshly harvested (wild and cultivated) E. -
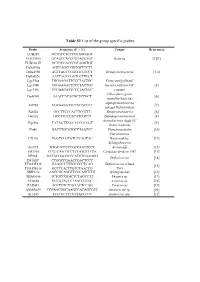
Table S1 List of the Group Specific Probes
Table S1 List of the group specific probes. Probe Sequence (5’ – 3’) Target References EUB338 GCTGCCTCCCGTAGGAGT EUB338 II GCAGCCACCCGTAGGTGT Bacteria [1][2] EUB338 III GCTGCCACCCGTAGGTGT Delta495a AGTTAGCCGGTGCTTCTT Delta495b AGTTAGCCGGCGCTTCCT Deltaproteobacteria [3,4] Delta495c AATTAGCCGGTGCTTCCT Lgc354a TGGAAGATTCCCTACTGC Firmicutes (Gram+ Lgc354b CGGAAGATTCCCTACTGC bacteria with low GC [5] Lgc354c CCGAAGATTCCCTACTGC content) Chloroflexi (green Gnsb941 AAACCACACGCTCCGCT [6] nonsulfur bacteria) Alphaproteobacteria Alf968 GGTAAGGTTCTGCGCGTT [7] (except Rickettsiales) Bet42a GCCTTCCCACTTCGTTT Betaproteobacteria [8] Gam2a GCCTTCCCACATCGTTT Gammaproteobacteria [8] Actinobacteria (high GC Hgc69a TATAGTTACCACCGCCGT [9] Gram+ bacteria) Pla46 GACTTGCATGCCTAATCC Planctomycetales [10] Flavobacteria, Cf319a TGGTCCGTGTCTCAGTAC Bacteroidetes, [11] Sphingobacteria Arc915 GTGCTCCCCCGCCAATTCCT Archaea [12] TM7905 CCGTCAATTCCTTTATGTTTTA Candidate division TM7 [13] DF988* GATACGACGCCCATGTCAAGGG Defluvicoccus [14] DF1020* CCGGCCGAACCGACTCCC TFO-DF218 GAAGCCTTTGCCCCTCAG Defluvicoccus related [15] TFO-DF618 GCCTCACTTGTCTAACCG TFO SBR9-1a AAGCGCAAGTTCCCAGGTTG Sphingomonas [16] THAU646 TCTGCCGTACTCTAGCCTT Thauera sp. [17] AZO644 GCCGTACTCTAGCCGTGC Azoarcus sp. [18] PAR651 ACCTCTCTCGAACTCCAG Paracoccus [19] AMAR839 CCGAACGGCAAGCCACAGCGTC Amaricoccus sp. [20] ACI145 TTTCGCTTCGTTATCCCC Acidovorax spp. [21] Table S2 Primers used in PCR and Sequencing. Primers Sequence (5’ – 3’) PCR 27f AGAGTTTGATCMTGGCTCAG 1492r TACGGYTACCTTGTTACGACTT T7f TAATACGACTCACTATAGGG -

Delajieldii, E. Falsen (EF) Group 13, EF Group 16, and Several Clinical Isolates, with the Species Acidovorax Facilis Comb
INTERNATIONAL JOURNAL OF SYSTEMATIC BACTERIOLOGY, OCt. 1990, p. 384-398 Vol. 40, No. 4 OO20-7713/90/040384-15$02.OO/O Copyright 0 1990, International Union of Microbiological Societies Acidovorax, a New Genus for Pseudomonas facilis, Pseudomonas delaJieldii, E. Falsen (EF) Group 13, EF Group 16, and Several Clinical Isolates, with the Species Acidovorax facilis comb. nov. , Acidovorax delaJieldii comb. nov., and Acidovorax temperans sp. nov. A. WILLEMS,l E. FALSEN,2 B. POT,l E. JANTZEN,3 B. HOSTE,l P. VANDAMME,' M. GILLIS,l* K. KERSTERS,l AND J. DE LEY' Laboratorium voor Microbiologie en Microbiele Genetica, Rijksuniversiteit, B-9000 Ghent, Belgium'; Culture Collection, Department of Clinical Bacteriology, University of Goteborg, ,5413 46 Goteborg, Sweden2; and Department of Methodology, National Institute of Public Health, N-0462 Oslo, Norway3 Pseudomonas facilis and Pseudomonas delafeldii are inappropriately assigned to the genus Pseudomonas. They belong to the acidovorans rRNA complex in rRNA superfamily I11 (i.e., the beta subclass of the Proteobacteria). The taxonomic relationships of both of these species, two groups of clinical isolates (E. Falsen [EF] group 13 and EF group 16), and several unidentified or presently misnamed strains were examined by using DNA:rRNA hybridization, numerical analyses of biochemical and auxanographic features and of fatty acid patterns, polyacrylamide gel electrophoresis of cellular proteins, and DNA:DNA hybridization. These organisms form a separate group within the acidovorans rRNA complex, and we -

Identification of Pseudomonas Species and Other Non-Glucose Fermenters
UK Standards for Microbiology Investigations Identification of Pseudomonas species and other Non- Glucose Fermenters Issued by the Standards Unit, Microbiology Services, PHE Bacteriology – Identification | ID 17 | Issue no: 3 | Issue date: 13.04.15 | Page: 1 of 41 © Crown copyright 2015 Identification of Pseudomonas species and other Non-Glucose Fermenters Acknowledgments UK Standards for Microbiology Investigations (SMIs) are developed under the auspices of Public Health England (PHE) working in partnership with the National Health Service (NHS), Public Health Wales and with the professional organisations whose logos are displayed below and listed on the website https://www.gov.uk/uk- standards-for-microbiology-investigations-smi-quality-and-consistency-in-clinical- laboratories. SMIs are developed, reviewed and revised by various working groups which are overseen by a steering committee (see https://www.gov.uk/government/groups/standards-for-microbiology-investigations- steering-committee). The contributions of many individuals in clinical, specialist and reference laboratories who have provided information and comments during the development of this document are acknowledged. We are grateful to the Medical Editors for editing the medical content. For further information please contact us at: Standards Unit Microbiology Services Public Health England 61 Colindale Avenue London NW9 5EQ E-mail: [email protected] Website: https://www.gov.uk/uk-standards-for-microbiology-investigations-smi-quality- and-consistency-in-clinical-laboratories -

Heterotrophic Plate Counts and Drinking-Water Safety
Heterotrophic Plate Counts and Drinking-water Safety The Significance of HPCs for Water Quality and Human Health Heterotrophic Plate Counts and Drinking-water Safety The Significance of HPCs for Water Quality and Human Health Edited by J. Bartram, J. Cotruvo, M. Exner, C. Fricker, A. Glasmacher Published on behalf of the World Health Organization by IWA Publishing, Alliance House, 12 Caxton Street, London SW1H 0QS, UK Telephone: +44 (0) 20 7654 5500; Fax: +44 (0) 20 7654 5555; Email: [email protected] www.iwapublishing.com First published 2003 World Health Organization 2003 Printed by TJ International (Ltd), Padstow, Cornwall, UK Apart from any fair dealing for the purposes of research or private study, or criticism or review, as permitted under the UK Copyright, Designs and Patents Act (1998), no part of this publication may be reproduced, stored or transmitted in any form or by any means, without the prior permission in writing of the publisher, or, in the case of photographic reproduction, in accordance with the terms of licences issued by the Copyright Licensing Agency in the UK, or in accordance with the terms of licenses issued by the appropriate reproduction rights organization outside the UK. Enquiries concerning reproduction outside the terms stated here should be sent to IWA Publishing at the address printed above. The publisher makes no representation, express or implied, with regard to the accuracy of the information contained in this book and cannot accept any legal responsibility or liability for errors or omissions that may be made. Disclaimer The opinions expressed in this publication are those of the authors and do not necessarily reflect the views or policies of the International Water Association, NSF International, or the World Health Organization.