Olabode Ope Samuel.Pdf (6.682Mb)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fish Bacterial Flora Identification Via Rapid Cellular Fatty Acid Analysis
Fish bacterial flora identification via rapid cellular fatty acid analysis Item Type Thesis Authors Morey, Amit Download date 09/10/2021 08:41:29 Link to Item http://hdl.handle.net/11122/4939 FISH BACTERIAL FLORA IDENTIFICATION VIA RAPID CELLULAR FATTY ACID ANALYSIS By Amit Morey /V RECOMMENDED: $ Advisory Committe/ Chair < r Head, Interdisciplinary iProgram in Seafood Science and Nutrition /-■ x ? APPROVED: Dean, SchooLof Fisheries and Ocfcan Sciences de3n of the Graduate School Date FISH BACTERIAL FLORA IDENTIFICATION VIA RAPID CELLULAR FATTY ACID ANALYSIS A THESIS Presented to the Faculty of the University of Alaska Fairbanks in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE By Amit Morey, M.F.Sc. Fairbanks, Alaska h r A Q t ■ ^% 0 /v AlA s ((0 August 2007 ^>c0^b Abstract Seafood quality can be assessed by determining the bacterial load and flora composition, although classical taxonomic methods are time-consuming and subjective to interpretation bias. A two-prong approach was used to assess a commercially available microbial identification system: confirmation of known cultures and fish spoilage experiments to isolate unknowns for identification. Bacterial isolates from the Fishery Industrial Technology Center Culture Collection (FITCCC) and the American Type Culture Collection (ATCC) were used to test the identification ability of the Sherlock Microbial Identification System (MIS). Twelve ATCC and 21 FITCCC strains were identified to species with the exception of Pseudomonas fluorescens and P. putida which could not be distinguished by cellular fatty acid analysis. The bacterial flora changes that occurred in iced Alaska pink salmon ( Oncorhynchus gorbuscha) were determined by the rapid method. -

Phenotypic and Genetic Diversity of Pseudomonads
PHENOTYPIC AND GENETIC DIVERSITY OF PSEUDOMONADS ASSOCIATED WITH THE ROOTS OF FIELD-GROWN CANOLA A Thesis Submitted to the College of Graduate Studies and Research In Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy In the Department of Applied Microbiology and Food Science University of Saskatchewan Saskatoon By Danielle Lynn Marie Hirkala © Copyright Danielle Lynn Marie Hirkala, November 2006. All rights reserved. PERMISSION TO USE In presenting this thesis in partial fulfilment of the requirements for a Postgraduate degree from the University of Saskatchewan, I agree that the Libraries of this University may make it freely available for inspection. I further agree that permission for copying of this thesis in any manner, in whole or in part, for scholarly purposes may be granted by the professor or professors who supervised my thesis work or, in their absence, by the Head of the Department or the Dean of the College in which my thesis work was done. It is understood that any copying or publication or use of this thesis or parts thereof for financial gain shall not be allowed without my written permission. It is also understood that due recognition shall be given to me and to the University of Saskatchewan in any scholarly use which may be made of any material in my thesis. Requests for permission to copy or to make other use of material in this thesis in whole or part should be addressed to: Head of the Department of Applied Microbiology and Food Science University of Saskatchewan Saskatoon, Saskatchewan, S7N 5A8 i ABSTRACT Pseudomonads, particularly the fluorescent pseudomonads, are common rhizosphere bacteria accounting for a significant portion of the culturable rhizosphere bacteria. -

Diaphorobacter Nitroreducens Gen. Nov., Sp. Nov., a Poly (3
J. Gen. Appl. Microbiol., 48, 299–308 (2002) Full Paper Diaphorobacter nitroreducens gen. nov., sp. nov., a poly(3-hydroxybutyrate)-degrading denitrifying bacterium isolated from activated sludge Shams Tabrez Khan and Akira Hiraishi* Department of Ecological Engineering, Toyohashi University of Technology, Toyohashi 441–8580, Japan (Received August 12, 2002; Accepted October 23, 2002) Three denitrifying strains of bacteria capable of degrading poly(3-hydroxybutyrate) (PHB) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) were isolated from activated sludge and characterized. All of the isolates had almost identical phenotypic characteristics. They were motile gram-negative rods with single polar flagella and grew well with simple organic com- pounds, as well as with PHB and PHBV, as carbon and energy sources under both aerobic and anaerobic denitrifying conditions. However, none of the sugars tested supported their growth. The cellular fatty acid profiles showed the presence of C16:1w7cis and C16:0 as the major com- ponents and of 3-OH-C10:0 as the sole component of hydroxy fatty acids. Ubiquinone-8 was de- tected as the major respiratory quinone. A 16S rDNA sequence-based phylogenetic analysis showed that all the isolates belonged to the family Comamonadaceae, a major group of b-Pro- teobacteria, but formed no monophyletic cluster with any previously known species of this fam- (DSM 13225؍) ily. The closest relative to our strains was an unidentified bacterium strain LW1 (99.9% similarity), reported previously as a 1-chloro-4-nitrobenzene degrading bacterium. DNA- DNA hybridization levels among the new isolates were more than 60%, whereas those between our isolates and strain DSM 13225 were less than 50%. -

Transfer of Several Phytopathogenic Pseudomonas Species to Acidovorax As Acidovorax Avenae Subsp
INTERNATIONALJOURNAL OF SYSTEMATICBACTERIOLOGY, Jan. 1992, p. 107-119 Vol. 42, No. 1 0020-7713/92/010107-13$02 .OO/O Copyright 0 1992, International Union of Microbiological Societies Transfer of Several Phytopathogenic Pseudomonas Species to Acidovorax as Acidovorax avenae subsp. avenae subsp. nov., comb. nov. , Acidovorax avenae subsp. citrulli, Acidovorax avenae subsp. cattleyae, and Acidovorax konjaci A. WILLEMS,? M. GOOR, S. THIELEMANS, M. GILLIS,” K. KERSTERS, AND J. DE LEY Laboratorium voor Microbiologie en microbiele Genetica, Rijksuniversiteit Gent, K.L. Ledeganckstraat 35, B-9000 Ghent, Belgium DNA-rRNA hybridizations, DNA-DNA hybridizations, polyacrylamide gel electrophoresis of whole-cell proteins, and a numerical analysis of carbon assimilation tests were carried out to determine the relationships among the phylogenetically misnamed phytopathogenic taxa Pseudomonas avenue, Pseudomonas rubrilineans, “Pseudomonas setariae, ” Pseudomonas cattleyae, Pseudomonas pseudoalcaligenes subsp. citrulli, and Pseudo- monas pseudoalcaligenes subsp. konjaci. These organisms are all members of the family Comamonadaceae, within which they constitute a separate rRNA branch. Only P. pseudoalcaligenes subsp. konjaci is situated on the lower part of this rRNA branch; all of the other taxa cluster very closely around the type strain of P. avenue. When they are compared phenotypically, all of the members of this rRNA branch can be differentiated from each other, and they are, as a group, most closely related to the genus Acidovorax. DNA-DNA hybridization experiments showed that these organisms constitute two genotypic groups. We propose that the generically misnamed phytopathogenic Pseudomonas species should be transferred to the genus Acidovorax as Acidovorax avenue and Acidovorax konjaci. Within Acidovorax avenue we distinguished the following three subspecies: Acidovorax avenue subsp. -

Activities and Prevalence of Proteobacteria Members Colonizing Echinacea Purpurea Fully Account for Macro- Phage Activation Exhibited by Extracts of This Botanical
1258 Original Papers Activities and Prevalence of Proteobacteria Members Colonizing Echinacea purpurea Fully Account for Macro- phage Activation Exhibited by Extracts of This Botanical Authors Mona H. Haron 1*, Heather L. Tyler 2, 3*, Nirmal D. Pugh 1, Rita M. Moraes1, Victor L. Maddox4, Colin R. Jackson 2, David S. Pasco1,5 Affiliations The affiliations are listed at the end of the article Key words Abstract highest potency for in vitro macrophage activa- l" Echinacea purpurea ! tion and were the most predominant taxa. Fur- l" Asteraceae Evidence supports the theory that bacterial com- thermore, the mean activity exhibited by the l" immunostimulatory munities colonizing Echinacea purpurea contrib- Echinacea extracts could be solely accounted for l" macrophage activation ute to the innate immune enhancing activity of by the activities and prevalence of Proteobacteria l" bacteria l" proteobacteria this botanical. Previously, we reported that only members comprising the plant-associated bacte- l" endophytes about half of the variation in in vitro monocyte rial community. The efficacy of E. purpurea mate- stimulating activity exhibited by E. purpurea ex- rial for use against respiratory infections may be tracts could be accounted for by total bacterial determined by the Proteobacterial community load within the plant material. In the current composition of this plant, since ingestion of bac- study, we test the hypothesis that the type of bac- teria (probiotics) is reported to have a protective teria, in addition to bacterial load, is necessary to effect against this health condition. fully account for extract activity. Bacterial com- munity composition within commercial and freshly harvested (wild and cultivated) E. -
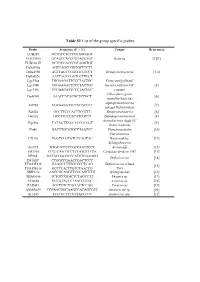
Table S1 List of the Group Specific Probes
Table S1 List of the group specific probes. Probe Sequence (5’ – 3’) Target References EUB338 GCTGCCTCCCGTAGGAGT EUB338 II GCAGCCACCCGTAGGTGT Bacteria [1][2] EUB338 III GCTGCCACCCGTAGGTGT Delta495a AGTTAGCCGGTGCTTCTT Delta495b AGTTAGCCGGCGCTTCCT Deltaproteobacteria [3,4] Delta495c AATTAGCCGGTGCTTCCT Lgc354a TGGAAGATTCCCTACTGC Firmicutes (Gram+ Lgc354b CGGAAGATTCCCTACTGC bacteria with low GC [5] Lgc354c CCGAAGATTCCCTACTGC content) Chloroflexi (green Gnsb941 AAACCACACGCTCCGCT [6] nonsulfur bacteria) Alphaproteobacteria Alf968 GGTAAGGTTCTGCGCGTT [7] (except Rickettsiales) Bet42a GCCTTCCCACTTCGTTT Betaproteobacteria [8] Gam2a GCCTTCCCACATCGTTT Gammaproteobacteria [8] Actinobacteria (high GC Hgc69a TATAGTTACCACCGCCGT [9] Gram+ bacteria) Pla46 GACTTGCATGCCTAATCC Planctomycetales [10] Flavobacteria, Cf319a TGGTCCGTGTCTCAGTAC Bacteroidetes, [11] Sphingobacteria Arc915 GTGCTCCCCCGCCAATTCCT Archaea [12] TM7905 CCGTCAATTCCTTTATGTTTTA Candidate division TM7 [13] DF988* GATACGACGCCCATGTCAAGGG Defluvicoccus [14] DF1020* CCGGCCGAACCGACTCCC TFO-DF218 GAAGCCTTTGCCCCTCAG Defluvicoccus related [15] TFO-DF618 GCCTCACTTGTCTAACCG TFO SBR9-1a AAGCGCAAGTTCCCAGGTTG Sphingomonas [16] THAU646 TCTGCCGTACTCTAGCCTT Thauera sp. [17] AZO644 GCCGTACTCTAGCCGTGC Azoarcus sp. [18] PAR651 ACCTCTCTCGAACTCCAG Paracoccus [19] AMAR839 CCGAACGGCAAGCCACAGCGTC Amaricoccus sp. [20] ACI145 TTTCGCTTCGTTATCCCC Acidovorax spp. [21] Table S2 Primers used in PCR and Sequencing. Primers Sequence (5’ – 3’) PCR 27f AGAGTTTGATCMTGGCTCAG 1492r TACGGYTACCTTGTTACGACTT T7f TAATACGACTCACTATAGGG -

Identification of Pseudomonas Species and Other Non-Glucose Fermenters
UK Standards for Microbiology Investigations Identification of Pseudomonas species and other Non- Glucose Fermenters Issued by the Standards Unit, Microbiology Services, PHE Bacteriology – Identification | ID 17 | Issue no: 3 | Issue date: 13.04.15 | Page: 1 of 41 © Crown copyright 2015 Identification of Pseudomonas species and other Non-Glucose Fermenters Acknowledgments UK Standards for Microbiology Investigations (SMIs) are developed under the auspices of Public Health England (PHE) working in partnership with the National Health Service (NHS), Public Health Wales and with the professional organisations whose logos are displayed below and listed on the website https://www.gov.uk/uk- standards-for-microbiology-investigations-smi-quality-and-consistency-in-clinical- laboratories. SMIs are developed, reviewed and revised by various working groups which are overseen by a steering committee (see https://www.gov.uk/government/groups/standards-for-microbiology-investigations- steering-committee). The contributions of many individuals in clinical, specialist and reference laboratories who have provided information and comments during the development of this document are acknowledged. We are grateful to the Medical Editors for editing the medical content. For further information please contact us at: Standards Unit Microbiology Services Public Health England 61 Colindale Avenue London NW9 5EQ E-mail: [email protected] Website: https://www.gov.uk/uk-standards-for-microbiology-investigations-smi-quality- and-consistency-in-clinical-laboratories -

Drinking Water Microflora Biofilms and Chlorine Susceptibility
University of Calgary PRISM: University of Calgary's Digital Repository Graduate Studies The Vault: Electronic Theses and Dissertations 2012-07-19 Drinking water microflora biofilms and chlorine susceptibility Schwering, Monika C. Schwering, M. C. (2012). Drinking water microflora biofilms and chlorine susceptibility (Unpublished master's thesis). University of Calgary, Calgary, AB. doi:10.11575/PRISM/25749 http://hdl.handle.net/11023/128 master thesis University of Calgary graduate students retain copyright ownership and moral rights for their thesis. You may use this material in any way that is permitted by the Copyright Act or through licensing that has been assigned to the document. For uses that are not allowable under copyright legislation or licensing, you are required to seek permission. Downloaded from PRISM: https://prism.ucalgary.ca UNIVERSITY OF CALGARY Drinking water microflora biofilms and chlorine susceptibility by Monika C. Schwering A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE DEPARTMENT OF BIOLOGICAL SCIENCE CALGARY, ALBERTA JULY, 2012 © Monika C. Schwering 2012 UNIVERSITY OF CALGARY FACULTY OF GRADUATE STUDIES The undersigned certify that they have read, and recommend to the Faculty of Graduate Studies for acceptance, a thesis entitled "Drinking Water Microflora Biofilms and Chlorine Susceptibility" submitted by Monika C. Schwering in partial fulfilment of the requirements of the degree of Master of Science. Supervisor, Dr. Howard Ceri, Department of Biological Sciences Interim Supervisor, Dr. Raymond J. Turner, Department of Biological Sciences Dr, Lisa M. Gieg, Department of Biological Sciences Dr. Marie Louie, Departments of Microbiology, Immunology and Infectious Diseases and Pathology and Laboratory Medicine Dr. -

Heterotrophic Plate Counts and Drinking-Water Safety
Heterotrophic Plate Counts and Drinking-water Safety The Significance of HPCs for Water Quality and Human Health Heterotrophic Plate Counts and Drinking-water Safety The Significance of HPCs for Water Quality and Human Health Edited by J. Bartram, J. Cotruvo, M. Exner, C. Fricker, A. Glasmacher Published on behalf of the World Health Organization by IWA Publishing, Alliance House, 12 Caxton Street, London SW1H 0QS, UK Telephone: +44 (0) 20 7654 5500; Fax: +44 (0) 20 7654 5555; Email: [email protected] www.iwapublishing.com First published 2003 World Health Organization 2003 Printed by TJ International (Ltd), Padstow, Cornwall, UK Apart from any fair dealing for the purposes of research or private study, or criticism or review, as permitted under the UK Copyright, Designs and Patents Act (1998), no part of this publication may be reproduced, stored or transmitted in any form or by any means, without the prior permission in writing of the publisher, or, in the case of photographic reproduction, in accordance with the terms of licences issued by the Copyright Licensing Agency in the UK, or in accordance with the terms of licenses issued by the appropriate reproduction rights organization outside the UK. Enquiries concerning reproduction outside the terms stated here should be sent to IWA Publishing at the address printed above. The publisher makes no representation, express or implied, with regard to the accuracy of the information contained in this book and cannot accept any legal responsibility or liability for errors or omissions that may be made. Disclaimer The opinions expressed in this publication are those of the authors and do not necessarily reflect the views or policies of the International Water Association, NSF International, or the World Health Organization. -

Complete Genome Sequence of the Phenanthrene-Degrading Soil Bacterium Delftia Acidovorans Cs1-4 Ameesha R
Shetty et al. Standards in Genomic Sciences (2015) 10:55 DOI 10.1186/s40793-015-0041-x EXTENDED GENOME REPORT Open Access Complete genome sequence of the phenanthrene-degrading soil bacterium Delftia acidovorans Cs1-4 Ameesha R. Shetty1, Vidya de Gannes2, Chioma C. Obi3, Susan Lucas4, Alla Lapidus5, Jan-Fang Cheng4, Lynne A. Goodwin6, Samuel Pitluck4, Linda Peters4, Natalia Mikhailova4, Hazuki Teshima6, Cliff Han6, Roxanne Tapia6, Miriam Land7, Loren J. Hauser7, Nikos Kyrpides4, Natalia Ivanova4, Ioanna Pagani4, Patrick S. G. Chain6, Vincent J Denef8, Tanya Woyke4 and William J. Hickey1* Abstract Polycyclic aromatic hydrocarbons (PAH) are ubiquitous environmental pollutants and microbial biodegradation is an important means of remediation of PAH-contaminated soil. Delftia acidovorans Cs1-4 (formerly Delftia sp. Cs1-4) was isolated by using phenanthrene as the sole carbon source from PAH contaminated soil in Wisconsin. Its full genome sequence was determined to gain insights into a mechanisms underlying biodegradation of PAH. Three genomic libraries were constructed and sequenced: an Illumina GAii shotgun library (916,416,493 reads), a 454 Titanium standard library (770,171 reads) and one paired-end 454 library (average insert size of 8 kb, 508,092 reads). The initial assembly contained 40 contigs in two scaffolds. The 454 Titanium standard data and the 454 paired end data were assembled together and the consensus sequences were computationally shredded into 2 kb overlapping shreds. Illumina sequencing data was assembled, and the consensus sequence was computationally shredded into 1.5 kb overlapping shreds. Gaps between contigs were closed by editing in Consed, by PCR and by Bubble PCR primer walks. -

Isolation and Characterization of a Novel Bacterial Strain From
F1000Research 2020, 9:656 Last updated: 01 JUN 2021 RESEARCH ARTICLE Isolation and characterization of a novel bacterial strain from a Tris-Acetate-Phosphate agar medium plate of the green micro-alga Chlamydomonas reinhardtii that can utilize common environmental pollutants as a carbon source [version 1; peer review: 3 approved] Mautusi Mitra 1, Kevin Manoap-Anh-Khoa Nguyen1,2, Taylor Wayland Box1, Jesse Scott Gilpin1, Seth Ryan Hamby1, Taylor Lynne Berry3,4, Erin Harper Duckett1 1Department of Biology, University of West Georgia, Carrollton, Georgia, 30118, USA 2Department of Mechanical Engineering, Kennesaw State University, Marietta, Georgia, 30060, USA 3Carrollton High School, Carrollton, Georgia, 30117, USA 4Department of Chemistry and Biochemistry, University of North Georgia, Dahlonega, Georgia, 30597, USA v1 First published: 29 Jun 2020, 9:656 Open Peer Review https://doi.org/10.12688/f1000research.24680.1 Latest published: 29 Jun 2020, 9:656 https://doi.org/10.12688/f1000research.24680.1 Reviewer Status Invited Reviewers Abstract Background: Chlamydomonas reinhardtii, a green micro-alga can be 1 2 3 grown at the lab heterotrophically or photo-heterotrophically in Tris- Phosphate-Acetate (TAP) medium which contains acetate as the sole version 1 carbon source. When grown in TAP medium, Chlamydomonas can 29 Jun 2020 report report report utilize the exogenous acetate in the medium for gluconeogenesis using the glyoxylate cycle, which is also present in many bacteria and 1. Dickson Aruhomukama , Makerere higher plants. A novel bacterial strain, LMJ, was isolated from a contaminated TAP medium plate of Chlamydomonas. We present our University, Kampala, Uganda work on the isolation and physiological and biochemical characterizations of LMJ. -

Phenotypic Variation and Molecular Signaling in the Interaction of the Rhizosphere Bacteria Acidovorax Sp
Phenotypic variation and molecular signaling in the interaction of the rhizosphere bacteria Acidovorax sp. N35 and Rhizobium radiobacter F4 with roots Dan Li Helmholtz Zentrum München Deutsches Forschuungszentrum für Gesundheit und Umwelt Abteilung Mikroben-Pflanzen Interaktionen Dissertation Zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften der Fakultät für Biologie der Ludwig-Maximilians-Universität München September 2010 1. Gutachter : Prof. Dr. Anton Hartmann 2. Gutachter: Prof. Dr. Kirsten Jung Eingereicht am: 23. September 2010 Tag der mündlichen Prüfung: 04. February 2011 To my family and to my Xu Contents Contents Abbreviations ........................................................................................................................ 4 1 Introduction ........................................................................................................................ 5 1.1 Plant-microorganisms interactions in the rhizosphere ................................................ 5 1.1.1 The rhizosphere .................................................................................................... 5 1.1.2 Effects of microorganisms on plants .................................................................... 6 1.1.3 Molecular microbial techniques to study microbe-plant interactions .................. 7 1.2 Cell-cell communication in bacteria............................................................................ 7 1.2.1 Quorum sensing in Gram-negative bacteria ........................................................