Life History Mediates the Trade-Offs Among Different Components Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Full of Beans: a Study on the Alignment of Two Flowering Plants Classification Systems
Full of beans: a study on the alignment of two flowering plants classification systems Yi-Yun Cheng and Bertram Ludäscher School of Information Sciences, University of Illinois at Urbana-Champaign, USA {yiyunyc2,ludaesch}@illinois.edu Abstract. Advancements in technologies such as DNA analysis have given rise to new ways in organizing organisms in biodiversity classification systems. In this paper, we examine the feasibility of aligning two classification systems for flowering plants using a logic-based, Region Connection Calculus (RCC-5) ap- proach. The older “Cronquist system” (1981) classifies plants using their mor- phological features, while the more recent Angiosperm Phylogeny Group IV (APG IV) (2016) system classifies based on many new methods including ge- nome-level analysis. In our approach, we align pairwise concepts X and Y from two taxonomies using five basic set relations: congruence (X=Y), inclusion (X>Y), inverse inclusion (X<Y), overlap (X><Y), and disjointness (X!Y). With some of the RCC-5 relationships among the Fabaceae family (beans family) and the Sapindaceae family (maple family) uncertain, we anticipate that the merging of the two classification systems will lead to numerous merged solutions, so- called possible worlds. Our research demonstrates how logic-based alignment with ambiguities can lead to multiple merged solutions, which would not have been feasible when aligning taxonomies, classifications, or other knowledge or- ganization systems (KOS) manually. We believe that this work can introduce a novel approach for aligning KOS, where merged possible worlds can serve as a minimum viable product for engaging domain experts in the loop. Keywords: taxonomy alignment, KOS alignment, interoperability 1 Introduction With the advent of large-scale technologies and datasets, it has become increasingly difficult to organize information using a stable unitary classification scheme over time. -
Threatened and Endangered Species List
Effective April 15, 2009 - List is subject to revision For a complete list of Tennessee's Rare and Endangered Species, visit the Natural Areas website at http://tennessee.gov/environment/na/ Aquatic and Semi-aquatic Plants and Aquatic Animals with Protected Status State Federal Type Class Order Scientific Name Common Name Status Status Habit Amphibian Amphibia Anura Gyrinophilus gulolineatus Berry Cave Salamander T Amphibian Amphibia Anura Gyrinophilus palleucus Tennessee Cave Salamander T Crustacean Malacostraca Decapoda Cambarus bouchardi Big South Fork Crayfish E Crustacean Malacostraca Decapoda Cambarus cymatilis A Crayfish E Crustacean Malacostraca Decapoda Cambarus deweesae Valley Flame Crayfish E Crustacean Malacostraca Decapoda Cambarus extraneus Chickamauga Crayfish T Crustacean Malacostraca Decapoda Cambarus obeyensis Obey Crayfish T Crustacean Malacostraca Decapoda Cambarus pristinus A Crayfish E Crustacean Malacostraca Decapoda Cambarus williami "Brawley's Fork Crayfish" E Crustacean Malacostraca Decapoda Fallicambarus hortoni Hatchie Burrowing Crayfish E Crustacean Malocostraca Decapoda Orconectes incomptus Tennessee Cave Crayfish E Crustacean Malocostraca Decapoda Orconectes shoupi Nashville Crayfish E LE Crustacean Malocostraca Decapoda Orconectes wrighti A Crayfish E Fern and Fern Ally Filicopsida Polypodiales Dryopteris carthusiana Spinulose Shield Fern T Bogs Fern and Fern Ally Filicopsida Polypodiales Dryopteris cristata Crested Shield-Fern T FACW, OBL, Bogs Fern and Fern Ally Filicopsida Polypodiales Trichomanes boschianum -

Modern System of Classification the System of Classification Is Used All
Modern System of Classification The system of classification is used all types of information i.e. morphology, anatomy, histological, biochemical and DNA analyses. The modern classifications are as follows: Tackhtazan, Cronquist, Angiosperm Phyllogeny Group (APG) etc. According to Arther Cronquist, Angiosperm or Magnoliophyta Division is classified into two Classes: A. Class- Magnoliopsida (Dicotyledon) B. Class-Liliopsida (Monocotyledon) A. Class Magnoliopsida (Dicotyledon) has six subclasses (S), 63 orders, 315 families, and around 165400 species. The class has following subclasses: S1- Magnoliidae This subclass contains 8 orders, 39 families and about 11000 species. They are most primitive angiosperms. The major characteristics of the members of Magnoliidae subclass are: Many parted well-developed perianth of tepals Differentiated into sepals and petals but sometimes apetalous The stamens are numerous and mature in a centripetal manner S2-Hamamelidae This subclass possesses 11 orders, 23 families and about 3400 species, it is the smallest subclass of dicots. The important characteristics of the subclass are: The perienth is absent or poorly developed Many are unisexual flower The mature fruit contains single seed S3-Caryophyllidae The subclass Caryophyllidae contains three orders, 14 families and about 11000 species. The subclass has following characteristics: Mostly herbaceous mostly succulents or halophytes Placentation is mostly free central or basal Pollens are tricoplate S4-Dilleniidae It contains 13 orders, 78 families, and about 24000 species. The important characteristics are Contain many woody species Centrifugal maturation of stamens and binucleate pollen S5-Rosidae The subclass has 18 orders, 113 families, and about 60000 species. It is the largest subclass among the dicotyledons. Characteristics The flower has numerous stamens that mature in centripetal sequence. -

CRONQUIST's SYSTEM of CLASSIFICATION
CRONQUIST’s SYSTEM OF CLASSIFICATION Contents • Introduction •Basis of Classification •Class Magnoliopsida •Class Liliopsida •Merits •De-Mertis •Principles of Classification Arthur Lyman John Cronquist (1919 -1992) was a United States botanist and a specialist on Compositae. He is considered one of the most influential botanists of the 20th century, largely due to his formulation of the Cronquist system OF Classification BASIS OF CLASSIFICATION This system of classification is an elaboration of Bessey’s system of classification and a refinement over Takhtajan’s system (1964), which is based on morphological, anatomical, embryological, palynological, serological, cytological, chemical as well as ultra structural evidences. According to Cronquist’s system of classification, the angiosperms have been divided into two Classes The Cronquist system is a taxonomic classification system of flowering plants. It was developed by Arthur Cronquist in his texts An Integrated System of Classification of Flowering Plants (1981) and The Evolution and Classification of Flowering Plants (1968; 2nd edition, 1988). • Cronquist's system places flowering plants into two broad classes, Magnoliopsida (dicotyledons) and Liliopsida (monocotyledons). Within these classes, related orders are grouped into subclasses. • The scheme is still widely used, in either the original form or in adapted versions, but some botanists are adopting the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. • The system as laid out in An Integrated System of Classification of Flowering Plants (1981) counts 321 families and 64 orders in class Magnoliopsida and 19 orders and 65 families in class Liliopsida • Class Magnoliopsida 1. Subclass Magnoliidae (mostly basal dicots)(8 Orders, 39 families) 2. -

Topic – Contemporary System of Classification 1 Cronquist and Takhtajan
Topic – Contemporary System of Classification 1 Cronquist and Takhtajan Sub: Botany Course- M. Sc.(semester ll) , Department of Botany Paper- MBOTCC-6 Taxonomy, Anatomy and Embryology Unit- I Rajlaxmi Singh Assistant Professor P. G. Department of Botany, Patna University, Patna-800005 Email id: [email protected] Arthur Cronquist Developed a comprehensive system of classification of angiosperms which deals particularly with the grouping of families into orders on a worldwise basis. • Discussed a wide range of characters important to phylogenetic classification (provided keys to bring various taxa in accordance to this system, provided charts showing relationship of orders). • Considered seed ferns (pteridosperms) as ancestors of angiosperms. • His important phylogenetic ideas about angiosperms are as following: 1. The earliest angiosperms were shrubs rather than trees. 2. The simple entire leaf is primitive than compound leaf. 3. Reticulate venation is primitive than parallel venation. 4. Stems with scattered vascular bundles are advanced in comprasion to stems with bundles in a ring. 5. There is evolutionary decrease in activity and area of cambium. 6. Primitive flowers are large and terminal. Dichasial and monochasial types are basic units of inflorescence and other types of inflorescence are derived from these. 7. The primitive flowers had numerous whorls. Aggregation and reduction, elaboration and differentiation occurred during evolution. 8. Unisexual flowers are derived from bisexual flowers. 9. Entomophily is primitive than anemophily. 10. Axile placentation is ancestral and other types are derived from it. 11. Anatropous condition of ovules is primitive and other types are derived from it. 12. Unitegmic condition is advanced than bitegmic. 13. Polygonum type of embryo sac is primitive (8-nucleate) than 4-nucleate embryo sac. -
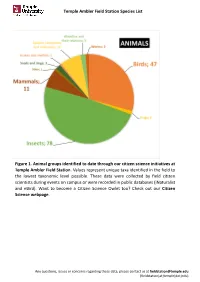
Temple Ambler Field Station Species List Figure 1. Animal Groups Identified to Date Through Our Citizen Science Initiatives at T
Temple Ambler Field Station Species List Figure 1. Animal groups identified to date through our citizen science initiatives at Temple Ambler Field Station. Values represent unique taxa identified in the field to the lowest taxonomic level possible. These data were collected by field citizen scientists during events on campus or were recorded in public databases (iNaturalist and eBird). Want to become a Citizen Science Owlet too? Check out our Citizen Science webpage. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List Figure 2. Plant diversity identified to date in the natural environments and designed gardens of the Temple Ambler Field Station and Ambler Arboretum. These values represent unique taxa identified to the lowest taxonomic level possible. Highlighted are 14 of the 116 flowering plant families present that include 524 taxonomic groups. A full list can be found in our species database. Cultivated specimens in our Greenhouse were not included here. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List database_title Temple Ambler Field Station Species List last_update 22October2020 description This database includes all species identified to their lowest taxonomic level possible in the natural environments and designed gardens on the Temple Ambler campus. These are occurrence records and each taxon is only entered once. This is an occurrence record, not an abundance record. IDs were performed by senior scientists and specialists, as well as citizen scientists visiting campus. -

The Dendroflora in the Jewish Cemetery
THE DENDROFLORA (TREES FUND) IN THE JEWISH CEMETERY The area of the Jewish cemetery has a plush and diversified dendroflora represented by many deciduous trees, coniferous trees and undergrowth. The trees are mainly concentrated along the sides of the pathways. The cemetery trees also forms a physical fence encircling the perimeter of the cemetery. Presently, the elaboration of a project is in progress with the aim to study the physical condition of the trees and plants inside the Jewish cemetery. Engaged in the realization of the project are professors of entomology and phytopathology of the Belgrade Faculty of Forestry. The task of this diploma paper is a survey of the Jewish Cemetery dendroflora. Exemplary types have been collected, established and described. It can be said of this area that it possesses an abundant dendrofund of exquisite esthetic value. During the process of on-site data gathering and research no evidence of any kind of disease was found except for a number of individual cases of tree desiccation. The conclusion of the paper should propose ways of further plant development within the Jewish cemetery or at least ways to preserve its current state. A TABLE OF THE TYPES OF TREES IN THE JEWISH CEMETERY No. Type of tree Life form Incidence Vitality 1. Acer negundo Tree frequent * Scientific classification Kingdom: Plantae Division: Magnoliophyta Class: Magnoliopsida Order: Sapindales Family: Sapindaceae Genus: Acer Species: A. negundo 2. Aesculus hippocastanum Tree frequent * Scientific classification Kingdom: Plantae Division: Magnoliophyta Class: Magnoliopsida Order: Sapindales Family: Sapindaceae Genus: Aesculus Species: A. hippocastanum 3. Ailanthus glandulosa Tree Very frequent * Scientific classification Kingdom: Plantae Division: Magnoliophyta Class: Magnoliopsida Order: Sapindales Family: Simaroubaceae Genus: Ailanthus Species: A. -

Michel Adanson
B.Sc. CORE COURCE X - PLANT SYSTEMATICS UNIT - 4 : CLASSIFICATION (PART-2) SOME OF THE MAJOR CONTRIBUTORS OF NATURAL SYSTEM OF CLASSIFICATION MICHEL ADANSON Michel Adanson (1727-1806), born at Aix-en-Provence, was an 18th-century French botanist and naturalist, who devised a natural system of classification and nomenclature of plants, based on all their physical characteristics, with an emphasis on families. His work on the baobabs trees results in their naming as Adansonia commemorating Adanson. In 1749 Adanson left for Senegal to spend four years as an employee with the Compagnie des Indes, a trading company. He returned with a large collection of plant specimens, some of which became part of the French royal collection under the supervision of the naturalist Georges Buffon; most of them now belong to the National Museum of Natural History in Paris. He published Histoire naturelle du Sénégal (1757), describing the flora of Senegal, and a survey of mollusks. Adanson’s Familles des plantes (1763) described his classification system for plants, which was much opposed by Carolus Linnaeus, the Swedish botanist who had proposed his own classification system based on the reproductive organs of plants. Adanson also introduced the use of statistical methods in botanical classification. Although Adanson was well known to European scientists, his system of classification was not widely successful, and it was superseded by the Linnaean system. Adansonia sp. (Baobabs tree) Adansonia digitata trees near Doranda College, Ranchi A.P. DE CANDOLLE Augustin Pyramus de Candolle (1778-1841) was a Swiss botanist. His main focus was botany, also contributed to related fields such as phytogeography, agronomy, paleontology, medical botany, and economic botany. -

Vascular Plants
Biodiversity Data Journal 7: e38687 doi: 10.3897/BDJ.7.e38687 Data Paper Biota from the coastal wetlands of Praia da Vitória (Terceira, Azores, Portugal): Part 4 – Vascular plants Rui B. Elias‡, Mariana R. Brito§, César M.M. Pimentel§, Elisabete C. Nogueira§, Paulo A. Borges‡ ‡ CE3C – Centre for Ecology, Evolution and Environmental Changes/Azorean Biodiversity Group and Universidade dos Açores - Faculdade de Ciências Agrárias e do Ambiente, Angra do Heroísmo, Portugal § LIFE CWR – LIFE project “Ecological Restoration and Conservation of Praia da Vitória Coastal Wet Green Infrastructures”, Praia da Vitória, Portugal Corresponding author: Rui B. Elias ([email protected]) Academic editor: Yasen Mutafchiev Received: 31 Jul 2019 | Accepted: 13 Sep 2019 | Published: 18 Oct 2019 Citation: Elias RB, Brito MR, Pimentel CM.M, Nogueira EC, Borges PA (2019) Biota from the coastal wetlands of Praia da Vitória (Terceira, Azores, Portugal): Part 4 – Vascular plants. Biodiversity Data Journal 7: e38687. https://doi.org/10.3897/BDJ.7.e38687 Abstract Background The data presented here come from field observations, carried out between 2014 and 2017, as part of a LIFE research project aiming to preserve and restore three coastal wetlands of Praia da Vitória (Terceira Island, Azores, Portugal) (LIFE-CWR). A total of 23 vascular plant species surveys were carried out in three sites: one for each semester in Paul da Praia da Vitória (PPV) and Paul da Pedreira do Cabo da Praia (PPCP); one for each semester (except in 2014) in Paul do Belo Jardim (PBJ). The main objectives were to determine the plant richness of the three sites and to monitor yearly variation on species composition. -

Angiosperm Phylogeny Group (APG) System
Angiosperm Phylogeny Group (APG) system The Angiosperm Phylogeny Group, or APG, refers to an informal international group of systematic botanists who came together to try to establish a consensus view of the taxonomy of flowering plants (angiosperms) that would reflect new knowledge about their relationships based upon phylogenetic studies. As of 2010, three incremental versions of a classification system have resulted from this collaboration (published in 1998, 2003 and 2009). An important motivation for the group was what they viewed as deficiencies in prior angiosperm classifications, which were not based on monophyletic groups (i.e. groups consisting of all the descendants of a common ancestor). APG publications are increasingly influential, with a number of major herbaria changing the arrangement of their collections to match the latest APG system. Angiosperm classification and the APG Until detailed genetic evidence became available, the classification of flowering plants (also known as angiosperms, Angiospermae , Anthophyta or Magnoliophyta ) was based on their morphology (particularly that of the flower) and their biochemistry (what kinds of chemical compound they contained or produced). Classification systems were typically produced by an individual botanist or by a small group. The result was a large number of such systems (see List of systems of plant taxonomy). Different systems and their updates tended to be favoured in different countries; e.g. the Engler system in continental Europe; the Bentham & Hooker system in Britain (particularly influential because it was used by Kew); the Takhtajan system in the former Soviet Union and countries within its sphere of influence; and the Cronquist system in the United States. -

Semester-II Taxonomy of Angiosperms BOTPG2202 NOMENCLATURE NOMENCLATURE Nomenclature Is Important in Order to Provide the Correct Name for a Plant
PG- Semester-II Taxonomy of Angiosperms BOTPG2202 NOMENCLATURE NOMENCLATURE Nomenclature is important in order to provide the correct name for a plant. The naming activity is under the control of the `International Codes of Botanical Nomenclature’ (ICBN) published by the `International Association of Plant Taxonomy’ (IAPT). The codes are revised at every `International Botanical Congress’ Plant Nomenclature •Botanical nomenclature is the formal, scientific naming of plants. It is related to, but distinct from taxonomy. Plant taxonomy is concerned with grouping and classifying plants; botanical nomenclature then provides names for the results of this process. •The starting point for modern botanical nomenclature is Linnaeus' Species Plantarum of 1753 (with exceptions). •Botanical nomenclature is governed by the International Code of Nomenclature for algae, fungi, and plants (ICN), which replaces the International Code of Botanical Nomenclature (ICBN). •Fossil plants are also covered by the code of nomenclature. What is meant by a scientific name? Scientific names are formal, universally accepted names, the rules and regulations of which for plants are provided by the ICN. Scientific names are always binomials (binary combinations), the genus name is always capitalized (as noun) and the second name of the binomial is the specific epithet. Binomial species names are always either italicized or underlined. For example. Quercus dumosa Nuttall or Quercus dumosa Nuttall Quercus is the genus name, dumosa is the specific epithet, Quercus dumosa is the species name and Nuttall is the author. State the reasons scientific names are advantageous over common names • Common names are names generally used by people within a limited geographic region that is not formally published and not governed by any rules. -

Taxonomy and Reproductive Biology of the Family Liliaceae of Bangladesh
TAXONOMY AND REPRODUCTIVE BIOLOGY OF THE FAMILY LILIACEAE OF BANGLADESH. A DISSERTATION SUBMITTED TO THE UNIVERSITY OF DHAKA FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BOTANY (PLANT TAXONOMY) FEBRUARY 2018 By SUMONA AFROZ Registration no. 102/2008-2009 Re-registration no. 54/2013-2014 Dedicated To My Beloved Parents, Husband And Respected Teachers DECLARATION I hereby declare that the work presented in this thesis entitled “Taxonomy and Reproductive Biology of the family Liliaceae of Bangladesh” is the result of my own investigation. I further declare that this thesis has not been submitted in any previous application for the award of any other academic degree in any university. All sources of information have been specifically acknowledged by referring to the authors. February, 2018 Sumona Afroz Author CERTIFICATE This is to certify that the research work presented in this dissertation entitled “Taxonomy and Reproductive Biology of the family Liliaceae of Bangladesh” is the outcome of the original work carried out by Sumona Afroz in the Plant Taxonomy, Ethnobotany and Herbal Medicine Laboratory, Department of Botany, University of Dhaka under my supervision. This is further certified that the style and contents of this dissertation is approved for submission in fulfillment of the requirements for the degree of Doctor of Philosophy in Botany (Plant Taxonomy). Prof. Dr. Md. Abul Hassan Supervisor Department of Botany University of Dhaka Dhaka-1000, Bangladesh ACKNOWLEDGEMENTS I sincerely feel that the Almighty 'Allah' has endowed me with immense blessings without which I could not have carried out this research work. The patience and aptitude that Allah has given me undoubtedly enabled to bring this task to a successful completion.