The Heydays of Mechanical Materialism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Classical Mechanics - Wikipedia, the Free Encyclopedia Page 1 of 13
Classical mechanics - Wikipedia, the free encyclopedia Page 1 of 13 Classical mechanics From Wikipedia, the free encyclopedia (Redirected from Newtonian mechanics) In physics, classical mechanics is one of the two major Classical mechanics sub-fields of mechanics, which is concerned with the set of physical laws describing the motion of bodies under the Newton's Second Law action of a system of forces. The study of the motion of bodies is an ancient one, making classical mechanics one of History of classical mechanics · the oldest and largest subjects in science, engineering and Timeline of classical mechanics technology. Branches Classical mechanics describes the motion of macroscopic Statics · Dynamics / Kinetics · Kinematics · objects, from projectiles to parts of machinery, as well as Applied mechanics · Celestial mechanics · astronomical objects, such as spacecraft, planets, stars, and Continuum mechanics · galaxies. Besides this, many specializations within the Statistical mechanics subject deal with gases, liquids, solids, and other specific sub-topics. Classical mechanics provides extremely Formulations accurate results as long as the domain of study is restricted Newtonian mechanics (Vectorial to large objects and the speeds involved do not approach mechanics) the speed of light. When the objects being dealt with become sufficiently small, it becomes necessary to Analytical mechanics: introduce the other major sub-field of mechanics, quantum Lagrangian mechanics mechanics, which reconciles the macroscopic laws of Hamiltonian mechanics physics with the atomic nature of matter and handles the Fundamental concepts wave-particle duality of atoms and molecules. In the case of high velocity objects approaching the speed of light, Space · Time · Velocity · Speed · Mass · classical mechanics is enhanced by special relativity. -

Leonhard Euler - Wikipedia, the Free Encyclopedia Page 1 of 14
Leonhard Euler - Wikipedia, the free encyclopedia Page 1 of 14 Leonhard Euler From Wikipedia, the free encyclopedia Leonhard Euler ( German pronunciation: [l]; English Leonhard Euler approximation, "Oiler" [1] 15 April 1707 – 18 September 1783) was a pioneering Swiss mathematician and physicist. He made important discoveries in fields as diverse as infinitesimal calculus and graph theory. He also introduced much of the modern mathematical terminology and notation, particularly for mathematical analysis, such as the notion of a mathematical function.[2] He is also renowned for his work in mechanics, fluid dynamics, optics, and astronomy. Euler spent most of his adult life in St. Petersburg, Russia, and in Berlin, Prussia. He is considered to be the preeminent mathematician of the 18th century, and one of the greatest of all time. He is also one of the most prolific mathematicians ever; his collected works fill 60–80 quarto volumes. [3] A statement attributed to Pierre-Simon Laplace expresses Euler's influence on mathematics: "Read Euler, read Euler, he is our teacher in all things," which has also been translated as "Read Portrait by Emanuel Handmann 1756(?) Euler, read Euler, he is the master of us all." [4] Born 15 April 1707 Euler was featured on the sixth series of the Swiss 10- Basel, Switzerland franc banknote and on numerous Swiss, German, and Died Russian postage stamps. The asteroid 2002 Euler was 18 September 1783 (aged 76) named in his honor. He is also commemorated by the [OS: 7 September 1783] Lutheran Church on their Calendar of Saints on 24 St. Petersburg, Russia May – he was a devout Christian (and believer in Residence Prussia, Russia biblical inerrancy) who wrote apologetics and argued Switzerland [5] forcefully against the prominent atheists of his time. -

Jeremiah Horrocks's Lancashire
Transits of Venus: New Views of the Solar System and Galaxy Proceedings IAU Colloquium No. 196, 2004 c 2004 International Astronomical Union D.W. Kurtz, ed. doi:10.1017/S1743921305001237 Jeremiah Horrocks’s Lancashire John K. Walton Department of History, University of Central Lancashire, Preston PR1 2HE, UK (email: [email protected]) Abstract. This paper sets Jeremiah Horrocks and Much Hoole in the context of Lancashire society on the eve of the English Civil War. It focuses on the complexities of what it was to be a “Puritan” in an environment where religious labels and conflicts mattered a great deal; it examines the economic circumstances of county and locality at the time, pointing out the extent to which (despite widespread and deep poverty) the county’s merchants were looking outwards to London, northern Europe and beyond; and it emphasizes that even in the apparently remote and rustic location of Much Hoole it was possible for Horrocks to sustain a scientific correspondence and to keep in touch with, and make his contribution to, developments on a much wider stage. This contribution is intended to complement Allan Chapman’s plenary lecture by devel- oping the local and Lancashire dimension to Jeremiah Horrocks’s astronomical activities at Much Hoole, less than three years before the outbreak of the great Civil War in 1642, which culminated in the execution of Charles I in 1649 and ushered in Britain’s only pe- riod of republican government, which lasted until the Restoration in 1660 (see Chapman 1994, to which this paper runs -
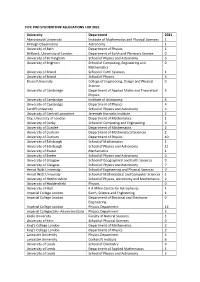
STFC Phd Studentship Allocation for 2021
STFC PHD STUDENTSHIP ALLOCATIONS FOR 2021 University Department 2021 Aberystwyth University Institute of Mathematics and Physical Sciences 1 Armagh Observatory Astronomy 1 University of Bath Department of Physics 1 Birkbeck, University of London Department of Earth and Planetary Science 0 University of Birmingham School of Physics and Astronomy 5 University of Brighton School of Computing, Engineering and 0 Mathematics University of Bristol School of Earth Sciences 1 University of Bristol School of Physics 3 Brunel University College of Engineering, Design and Physical 0 Science University of Cambridge Department of Applied Maths and Theoretical 5 Physics University of Cambridge Institute of Astronomy 6 University of Cambridge Department of Physics 4 Cardiff University School of Physics and Astronomy 4 University of Central Lancashire Jeremiah Horrocks Institute 2 City, University of London Department of Mathematics 1 University of Derby School of Computing and Engineering 0 University of Dundee Department of Mathematics 2 University of Durham Department of Mathematical Sciences 2 University of Durham Department of Physics 10 University of Edinburgh School of Mathematics 1 University of Edinburgh School of Physics and Astronomy 11 University of Exeter Mathematics 1 University of Exeter School of Physics and Astronomy 2 University of Glasgow School of Geographical and Earth Sciences 0 University of Glasgow School of Physics and Astronomy 7 Heriot Watt University School of Engineering and Physical Sciences 0 Heriot Watt University School -

Appendix A: Educational Resources in Astronomy
Appendix A: Educational Resources in Astronomy A.I Planetariums, Museums, and Exhibits A.I.I Planetariums and Museums in the United Kingdom England - AAC Planetarium, Amateur Astronomy Centre, Bacup Road, Clough Bank, Tod morden, Lancs. OLl4 7HW. Tel: 0706816964. - British Museum, Great Russell Street, London WC1B 3DG; Tel: 071-323 8395 ext. 395. Astronomical clocks. - British Museum (Natural History), Cromwell Road, South Kensington, London SW7 5BD; Tel: 071-938 9123. Extensive meteorite collection. - Caird Planetarium, Old Royal Observatory, Greenwich, London SE 10. - William Day Planetarium, Plymouth Polytechnic, School of Maritime Studies, Ply- mouth PL4 8AA. Tel: 0752 264666. - Electrosonic Ltd., 815 Woolwich Road, London SE7 8LT. - Greenwich Planetarium, South Building, Greenwich Park, Greenwich, London SE 10. Tel: 081-858 1167. - William Herschel House and Museum, 19 New King Street, Bath, BA1 2Bl. Con tact: Dr. A.V. Sims, 30 Meadow Park, Bathford, Bath; Tel: 0225 859529. Open Mar-Oct daily 2-5 pm, Nov-Feb Sundays only, 2-5 pm. - lodrell Bank Planetarium and Visitor Center, Lower Withington, Nr. Macclesfield, Cheshire SK11 9DL; Tel: 0477 71339. - Kings Observatory, Kew, Old Deer Park, Richmond, Surrey TW9 2AZ. - University of Leicester, The Planetarium, Department of Astronomy, University Road, Leicester LEI 7RH; Tel: 0533 522522. - Liverpool Museum Planetarium, William Brown Street, Liverpool, Merseyside L3 8EN. Tel: 051-2070001 ext. 225. - London Planetarium, Marylebone Road, London NW1 5LR; Tel: 071-486 1121 (9:30--5:30), 071-486 1121 (recording). - City of London Polytechnic, The Planetarium, 100 Minories, Tower Hill, London EC3N BY. 071-283 1030. - London Schools Planetarium, John Archer School Building, Wandsworth Rd., Sutherland Grove, London SW18; Tel: 081-788 4253. -

Kiss of the Goddess | the Economist
5/9/2016 Kiss of the goddess | The Economist Transits of Venus Kiss of the goddess Venus will soon cross the face of the sun, and astronomers around the world will have a party May 27th 2004 | From the print edition FOR about six hours on Tuesday June 8th, the sun will be fainter than usual. There is no need to panic. It will fade by a mere 0.1%. Only a diligent observer with the correct viewing equipment will notice something odd: there will be a small, dark dot on the disc of the sun, like a solitary pea on a yellow dinner plate. The pea is Venus, passing directly between Earth and the sun for the first time since 1882. Insignificant though it may seem, this rare celestial event, a “transit of Venus”, was once thought a key to understanding the universe. Two and a half centuries ago, countries dispatched astronomers on risky and expensive expeditions to observe transits from far-flung points across the globe. By doing this, they hoped to make a precise measurement of the distance to the sun and thus acquire an accurate yardstick by which the distance to everything else in the solar system could be measured. Though this quest fell short of its goal, it did produce a much better estimate of that distance. It also produced some interesting spin-offs, such as the invention of the movie camera and the discovery of Australia's Great Barrier Reef. In any case, astronomers—both professional and amateur—are eagerly awaiting the forthcoming transit so that they can commune with the shades of their predecessors. -

PDF Click to View Or Download
Learning Outcomes in Focus Contextual strand: PW3 Students should be able to investigate patterns and relationships between physical observables. INVESTIGATE: Observe, study or make detailed and systematic examination, in order to establish facts and reach new conclusions Nature of science: NoS1 Students should be able to appreciate how scientists work and how scientific ideas are modified over time. APPRECIATE: Have a practical understanding of Learning Intentions As this activity relates to many learning outcomes, including those above, be sure to decide on the specific Learning intentions you have for your class if they are to engage with this activity. Introduction and Teacher Background Students will use a light sensor at the beginning of this activity. A light sensor is an electronic device used to detect light. They usually consist of a photoelectric cell which converts light energy into electrical energy. (You might see links to PW5 and PW6). While the unit LUX is in fact itself a derived unit (Lumen m-2), it is a measure of the light intensity as perceived by the human eye and so students will explore light intensity in lux as a physical observable and investigate its relationship to distance from a source and varied obstacles. In the second half of this activity students will try to apply what they have learned to analyse data from the NASA Kepler mission. While in the initial part of the activity variation in light intensity was achieved by allowing a pendulum to swing over and back in front of a light source, a similar pattern, including regular drops in light intensity, would also be achieved as a planet rotated around a star. -

Jeremiah Horrocks and the Transits of Venus Editorial
Issue 5 – November 2004 Editorial David Rayner Welcome to the fifth issue of the is compiling a CD record of the updated with news and events. SHA Newsletter. As the eagle-eyed English Mechanic and World of Take a look - you'll be surprised at of you will already have noticed, this Science magazine, a tribute from the changes! issue carries the SHA logo and Peredur Williams on the centenary banner for the first time. The of the the interpretation of the P Finally, a reminder that articles and editorial team hopes you like the new Cygni line profile and Clive letters for the Newsletter are always appearance. Davenhall's revisit to the welcome. Addresses and details are Lancashire village of Much Hoole to be found on the back page. As well as the usual SHA news and to study transits of Venus old and Please don't think we need an in features this bumper-size issue new. depth piece of work, in fact from contains an interesting item from the point of view of compiling the Mike Frost on the eclipse of 1737 Members who have access to the Newsletter, snippets are really as recalled by records uncovered in Internet may like to know that our useful for fill-ins. All contributions the Warwickshire county archives, website www.shastro.org.uk/ has will be gratefully received! a cry of help from Eric Hutton who been reworked and is now regularly Jeremiah Horrocks and the transits of Venus In 1639 Jeremiah Horrocks made the first observations of a transit of Venus. -

Jeremiah Horrocks Date of Birth, Parentage and Family Associations
JEREMIAH HORROCKS DATE OF BIRTH, PARENTAGE AND FAMILY ASSOCIATIONS BY S. B. GAYTHORPE, F.R.A.S. fY man, however famous, for whom the usual official records of existence are lacking, may well seem but a rather nebulous Aperson. Such, unfortunately, is the position with regard to Jeremiah Horrocks, the seventeenth-century Lancashire astronomer famous for his prediction and observation of the first Transit of Venus ever witnessed. Despite the most diligent inquiries no record of his baptism or burial has so far been found, and it is not even known whether the reason is that the registers in question are missing, or that a search has not yet been made in the right place or in the correct year. Without some definite starting-point it may well seem hopeless to pursue the inquiry, but the following results of recent research may suggest one or two lines for further investigation. I DATE OF BIRTH One of the very few remaining original official records in which Horrock's name is found is the Matriculation Register of Cambridge University. Under the date 5 July 1632 this book records the names of twenty-one candidates, arranged in three groups, viz.: three fellow commoners, ten pensioners, and eight sizars (two of whom are elsewhere later described as pensioners), who were then sworn before the vice-chancellor, Thomas Comber, professor of Divinity in the University, in the presence of Masters -. Turwhitt and Lionel Gatford, proctors, and James Tabor, registrary. The names in each group do not appear to be arranged in any particular order, either of rank, or seniority, whether of age or duration of residence, so we may perhaps assume that they merely represent the order in which the candidates happened to be standing at the time or the order in which their names had been sent in to the registrary by the prelectors. -

Transit Tracks Transit Tracks P
Investigation: Transit Tracks Transit Tracks p. 1 Students will be able to • describe a transit and the conditions when a transit may be seen Materials and Preparation • describe how a planet’s size and distance from its star affects the • Clip-on lamp with frosted spherically behavior of transits shaped low wattage (25 W maximum) • interpret graphs of brightness vs time to deduce information about bulb. planet-star systems. • 4 beads, various sizes (3-12mm) and colors on threads of various lengths (20-100cm). A. What is a transit? For best results, use black thread. 1. Introduce students to the concept of transits by reading a short • “Transit Light Curves” - one set per group account of the first transit observation by Jeremiah Horrocks (http:// of 2-5 students kepler.nasa.gov/ed/lc/horrocks.html) • Worksheets: 1 per student or 1 per group 2. Demonstrate a transit by positioning the clip-on lamp at a height • Account of Jeremiah Horrocks observations between standing eye-level and seated eye-level. Swing the larg- of the transit of Venus from http://kepler. est bead on a thread in a circle around the lamp, with the lamp at nasa.gov/ed/lc the center in the plane of the orbit. Tell the class that the light bulb • Optional: light sensor and computer with represents a star and the bead a planet; the planet is orbiting its star, sensor interface and graphing function. like the Earth or Venus orbit the Sun. • Optional: Johannes Kepler information: a. With students seated, ask if anyone can see the bead go directly http://kepler.nasa.gov/Mission/ in front of the star. -

A Calendar of Mathematical Dates January
A CALENDAR OF MATHEMATICAL DATES V. Frederick Rickey Department of Mathematical Sciences United States Military Academy West Point, NY 10996-1786 USA Email: fred-rickey @ usma.edu JANUARY 1 January 4713 B.C. This is Julian day 1 and begins at noon Greenwich or Universal Time (U.T.). It provides a convenient way to keep track of the number of days between events. Noon, January 1, 1984, begins Julian Day 2,445,336. For the use of the Chinese remainder theorem in determining this date, see American Journal of Physics, 49(1981), 658{661. 46 B.C. The first day of the first year of the Julian calendar. It remained in effect until October 4, 1582. The previous year, \the last year of confusion," was the longest year on record|it contained 445 days. [Encyclopedia Brittanica, 13th edition, vol. 4, p. 990] 1618 La Salle's expedition reached the present site of Peoria, Illinois, birthplace of the author of this calendar. 1800 Cauchy's father was elected Secretary of the Senate in France. The young Cauchy used a corner of his father's office in Luxembourg Palace for his own desk. LaGrange and Laplace frequently stopped in on business and so took an interest in the boys mathematical talent. One day, in the presence of numerous dignitaries, Lagrange pointed to the young Cauchy and said \You see that little young man? Well! He will supplant all of us in so far as we are mathematicians." [E. T. Bell, Men of Mathematics, p. 274] 1801 Giuseppe Piazzi (1746{1826) discovered the first asteroid, Ceres, but lost it in the sun 41 days later, after only a few observations. -

Transits of Venus: New Views of the Solar System and Galaxy
INTERNATIONAL ASTRONOMICAL UNION UNION ASTRONOMIQUE INTERNATIONALE "I TRANSITS OF VENUS: NEW VIEWS OF THE SOLAR SYSTEM AND GALAXY PROCEEDINGS OF THE 196th COLLOQUIUM OF THE INTERNATIONAL ASTRONOMICAL UNION HELD IN PRESTON, LANCASHIRE, UNITED KINGDOM 7-11 JUNE 2004 Edited by D. W. KURTZ Centre for Astrophysics, University of Central Lancashire, Preston, UK CAMBRIDGE UNIVERSITY PRESS V Table of Contents Preface ix Organizing committee xii Conference photograph xiii Conference participants xvi Part 1. TRANSITS OF VENUS: HISTORY, RESULTS AND LEGACY Chairs: Steve Dick & Mary Bru'ck Jeremiah Horrocks, William Crabtree, and the Lancashire observations of the tran- sit of Venus of 1639 [Keynote talk] 3 Allan Chapman ~\ Jeremiah Horrocks's Lancashire 27 John K. Walton William Crabtree's Venus transit observation 34 Nicholas Kollerstrom Venus transits - A French view 41 Suzanne Debarbat James Cook's 1769 transit of Venus expedition to Tahiti 52 Wayne Orchiston v~ Observations of the 1761 and 1769 transits of Venus from Batavia (Dutch East Indies) 67 Robert H. van Gent The 1761 transit of Venus dispute between Audiffredi and Pingre 74 Luisa Pigatto Observations of planetary transits made in Ireland in the 18th Century and the development of astronomy in Ireland 87 C. J. Butler The American transit of Venus expeditions of 1874 and 1882 100 Steven J. Dick The Mexican expedition to observe the 8 December 1874 transit of Venus in Japan 111 Christine Allen Maya observations of 13th-century transits of Venus? 124 Jesus Galindo Trejo and Christine Allen Lord Lindsay's expedition to Mauritius in 1874 138 M. T. Briick ' vi Contents Why did other European astronomers not see the December 1639 transit of Venus? 146 David W.