Annual Report 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

View a Copy of This Licence, Visit Tivecommons.Org/Licenses/By/4.0
Katakami et al. Cardiovasc Diabetol (2020) 19:110 https://doi.org/10.1186/s12933-020-01079-4 Cardiovascular Diabetology ORIGINAL INVESTIGATION Open Access Tofoglifozin does not delay progression of carotid atherosclerosis in patients with type 2 diabetes: a prospective, randomized, open-label, parallel-group comparative study Naoto Katakami1,2* , Tomoya Mita3, Hidenori Yoshii4, Toshihiko Shiraiwa5, Tetsuyuki Yasuda6, Yosuke Okada7, Keiichi Torimoto7, Yutaka Umayahara8, Hideaki Kaneto9, Takeshi Osonoi10, Tsunehiko Yamamoto11, Nobuichi Kuribayashi12, Kazuhisa Maeda13, Hiroki Yokoyama14, Keisuke Kosugi15, Kentaro Ohtoshi16, Isao Hayashi17, Satoru Sumitani18, Mamiko Tsugawa19, Kayoko Ryomoto20, Hideki Taki21, Tadashi Nakamura22, Satoshi Kawashima23, Yasunori Sato24, Hirotaka Watada3 and Iichiro Shimomura1 on behalf of the UTOPIA study investigators Abstract Background: This study aimed to investigate the preventive efects of tofoglifozin, a selective sodium-glucose cotransporter 2 (SGLT2) inhibitor, on atherosclerosis progression in type 2 diabetes (T2DM) patients without apparent cardiovascular disease (CVD) by monitoring carotid intima-media thickness (IMT). Methods: This prospective, randomized, open-label, blinded-endpoint, multicenter, parallel-group, comparative study included 340 subjects with T2DM and no history of apparent CVD recruited at 24 clinical units. Subjects were randomly allocated to either the tofoglifozin treatment group (n 169) or conventional treatment group using drugs other than SGLT2 inhibitors (n 171). Primary outcomes were changes= in mean and maximum common carotid IMT measured by echography during= a 104-week treatment period. Results: In a mixed-efects model for repeated measures, the mean IMT of the common carotid artery (mean- IMT-CCA), along with the right and left maximum IMT of the CCA (max-IMT-CCA), signifcantly declined in both the tofoglifozin ( 0.132 mm, SE 0.007; 0.163 mm, SE 0.013; 0.170 mm, SE 0.020, respectively) and the control group ( 0.140 mm,− SE 0.006; 0.190 mm,− SE 0.012; 0.190 mm,− SE 0.020, respectively). -

OSI Pharmaceuticals and AVEO Pharmaceuticals Expand Oncology Discovery and Translational Research Collaboration
OSI Pharmaceuticals and AVEO Pharmaceuticals Expand Oncology Discovery and Translational Research Collaboration July 21, 2009 9:24 PM ET MELVILLE, NY and CAMBRIDGE, Mass., July 21, 2009 – OSI Pharmaceuticals, Inc. (NASDAQ:OSIP) and AVEO Pharmaceuticals, Inc. today announced that they have expanded the drug discovery and translational research collaboration announced in October of 2007. The alliance is anchored around developing molecular targeted therapies to target the underlying mechanisms of epithelial-mesenchymal transition (EMT) in cancer and to develop proprietary datasets of associated patient selection biomarkers to support OSI’s targeted medicine pipeline. EMT is a process of emerging significance in tumor development and disease progression and the focal point of OSI’s proprietary oncology research efforts. The companies are expanding their efforts to validate cancer targets and to deploy key elements of AVEO’s proprietary translational research platform in support of OSI’s clinical development programs. Under the terms of the expanded discovery and research agreement, OSI will pay AVEO a total of $20 million at closing, $5 million of which is an upfront cash payment and $15 million of which is the purchase of additional equity in AVEO. OSI will also provide AVEO research funding over the next two years to support the collaboration, and the potential to achieve additional royalties and milestones. In return, OSI will immediately receive rights beyond the original collaboration including rights to additional EMT targets (including up to 4 antibody targets) and increased access to AVEO technology (i.e. tumor models, archives and biomarkers). OSI is also acquiring non-exclusive access to AVEO’s proprietary bioinformatics platform. -

IPR2016-01284 Patent 6,900,221 B1 ______
[email protected] Paper No. 49 571.272.7822 Filed: January 8, 2018 UNITED STATES PATENT AND TRADEMARK OFFICE ____________________ BEFORE THE PATENT TRIAL AND APPEAL BOARD ____________________ APOTEX INC., APOTEX CORP., APOTEX PHARMACEUTICALS HOLDINGS INC., AND APOTEX HOLDINGS, INC., Petitioner, v. OSI PHARMACEUTICALS LLC, Patent Owner. ____________________ Case IPR2016-01284 Patent 6,900,221 B1 ____________ Before LORA M. GREEN, RAMA G. ELLURU, and ZHENYU YANG, Administrative Patent Judges. GREEN, Administrative Patent Judge. FINAL WRITTEN DECISION Determining That Claims 44‒46 and 53 Are Shown to Be Unpatentable 35 U.S.C. § 318(a) and 37 C.F.R. § 42.73 IPR2016-01284 Patent 6,900,221 B1 I. INTRODUCTION Apotex Inc., Apotex Corp., Apotex Pharmaceuticals Holdings Inc., and Apotex Holdings, Inc., (“Apotex” or “Petitioner”) filed a Petition requesting an inter partes review of claims 44–47 and 53 of U.S. Patent No. 6,900,221 B1 (Ex. 1001, “the ’221 patent”). Paper 3 (“Pet.”). OSI Pharmaceuticals LLC1 (“OSI” or “Patent Owner”) filed a Preliminary Response to the Petition.2 Paper 7 (“Prelim. Resp.”). We determined that the information presented in the Petition and the Preliminary Response demonstrated that there was a reasonable likelihood that Petitioner would prevail in challenging claims 44–47 and 53 as unpatentable under 35 U.S.C. § 103(a). Pursuant to 35 U.S.C. § 314, we instituted trial on January 9, 2017, as to all of the challenged claims of the ’221 patent. Paper 8 (“Institution Decision” or “Dec. Inst.”). On February 8, 2017, the parties filed a Joint Motion to Limit Petition Under 37 C.F.R. -

Ranking of Stocks by Market Capitalization(As of End of Mar.2020)
Ranking of Stocks by Market Capitalization(As of End of Mar.2020) 1st Section Rank Code Issue Market Capitalization \100mil. 1 7203 TOYOTA MOTOR CORPORATION 212,127 2 9437 NTT DOCOMO,INC. 112,630 3 6861 KEYENCE CORPORATION 84,709 4 6758 SONY CORPORATION 81,786 5 9984 SoftBank Group Corp. 79,162 6 9433 KDDI CORPORATION 75,136 7 4519 CHUGAI PHARMACEUTICAL CO.,LTD. 69,960 8 9432 NIPPON TELEGRAPH AND TELEPHONE CORPORATION 68,006 9 9434 SoftBank Corp. 65,799 10 7974 Nintendo Co.,Ltd. 54,787 11 8306 Mitsubishi UFJ Financial Group,Inc. 54,735 12 4568 DAIICHI SANKYO COMPANY,LIMITED 52,707 13 4502 Takeda Pharmaceutical Company Limited 52,146 14 4661 ORIENTAL LAND CO.,LTD. 50,261 15 6098 Recruit Holdings Co.,Ltd. 47,419 16 9983 FAST RETAILING CO.,LTD. 46,873 17 7182 JAPAN POST BANK Co.,Ltd. 44,865 18 4063 Shin-Etsu Chemical Co.,Ltd. 44,707 19 7267 HONDA MOTOR CO.,LTD. 44,017 20 4452 Kao Corporation 42,560 21 6367 DAIKIN INDUSTRIES,LTD. 38,603 22 6981 Murata Manufacturing Co.,Ltd. 36,980 23 8058 Mitsubishi Corporation 36,436 24 8316 Sumitomo Mitsui Financial Group,Inc. 36,018 25 9022 Central Japan Railway Company 35,679 26 8001 ITOCHU Corporation 35,541 27 8766 Tokio Marine Holdings,Inc. 35,145 28 7741 HOYA CORPORATION 34,808 29 6594 NIDEC CORPORATION 33,433 30 8035 Tokyo Electron Limited 32,000 31 3382 Seven & I Holdings Co.,Ltd. 31,699 32 7751 CANON INC. 31,463 33 8411 Mizuho Financial Group,Inc. -

Press Release
Press Release Company name: DAIICHI SANKYO COMPANY, LIMITED Representative: Sunao Manabe, Representative Director, President and CEO (Code no.: 4568, First Section, Tokyo Stock Exchange) Please address inquiries to Junichi Onuma, Vice President, Corporate Communications Department Telephone: +81-3-6225-1126 https://www.daiichisankyo.com Daiichi Sankyo Announces Transfer from Astellas Pharma of Three Products in Asia Tokyo, Japan (October 15, 2019) – Daiichi Sankyo Company, Limited (hereafter, Daiichi Sankyo) today announced that it agreed with Astellas Pharma Inc. (hereafter, Astellas Pharma) that Astellas Pharma local subsidiaries companies in six Asian countries will transfer three products to Daiichi Sankyo. The products to be transferred and the countries where they are sold are as follows. Product Korea China Taiwan Thailand Philippines Indonesia [generic name (brand name)] Ramosetron Antiemetic ○ ○ ○ ○ (Nasea) Nicardipine Anti- ○ ○ ○ (Perdipine) hypertensive Barnidipine Anti- ○ (Oldeca) hypertensive ○ indicate the countries where the products are sold The antiemetics are expected to have synergistic effects with mirogabalin and the cancer drugs that Daiichi Sankyo is currently developing in Asia, and the two antihypertensives are expected to effectively utilize Daiichi Sankyo’s current infrastructures in combination with its cardiovascular products, such as olmesartan and edoxaban. The total net sales of Astellas Pharma's three products in fiscal year 2018 were approximately 5.0 billion yen. 1 Daiichi Sankyo will take over the rights -

In the United States District Court for the District of Delaware
Case 1:15-cv-01063-UNA Document 1 Filed 11/17/15 Page 1 of 17 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE OSI PHARMACEUTICALS, LLC, PFIZER ) INC., and GENENTECH, INC., ) ) Plaintiffs, ) ) v. ) C.A. No. ) BRECKENRIDGE PHARMACEUTICAL, ) INC. and NATCO PHARMA LTD., ) ) Defendants. ) COMPLAINT FOR PATENT INFRINGEMENT Plaintiffs OSI Pharmaceuticals, LLC (“OSI”), Pfizer Inc. (“Pfizer”) and Genentech, Inc. (“Genentech”) (collectively, “Plaintiffs”), by their undersigned attorneys, bring this action against Defendant Breckenridge Pharmaceutical Inc., (“Breckenridge”), and Natco Pharma Ltd. (“Natco”) (collectively, “Breckenridge/Natco”) for patent infringement and allege as follows: NATURE OF THE ACTION 1. This is an action for patent infringement under the patent laws of the United States, Title 35 of the United States Code, the Federal Food, Drug, and Cosmetic Act, Title 21 of the United States Code, and the Declaratory Judgment Act, 28 U.S.C. §§ 2201 et seq., arising from Breckenridge/Natco’s filing of an Abbreviated New Drug Application No. 208488 (“Breckenridge/Natco ANDA” or “ANDA No. 208488”) with the United States Food and Drug Administration (“FDA”) seeking approval to commercially market generic versions of TARCEVA® (“Breckenridge/Natco’s Proposed Generic Products”) prior to the expiration of certain patents that relate to that product, United States Reissued Patent No. RE 41,065 (“the RE ‘065 patent”), and United States Patent No. 6,900,221 (“the ‘221 patent”). Case 1:15-cv-01063-UNA Document 1 Filed 11/17/15 Page 2 of 17 PageID #: 2 THE PARTIES 2. Plaintiff OSI is a limited liability company organized and existing under the laws of the State of Delaware, with a principal place of business at 1 Astellas Way, Northbrook, IL 60062. -

17TH ANNUAL PHARMACEUTICAL and MEDICAL DEVICE COMPLIANCE CONGRESS Office of Inspector General Update
17TH ANNUAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS Office of Inspector General Update Mary E. Riordan, Senior Counsel Office of Counsel to the Inspector General October 19, 2016 AGENDA FOR TODAY Update on recent enforcement activity Update on OIG reports and other publications Lessons/suggestions for consideration ISSUES IN RECENT SETTLEMENTS Settlements addressed a variety of areas: Kickback issues Marketing and sales issues Medicaid drug rebate program issues Drug price reporting issues FALSE CLAIM ACT SETTLEMENTS Kickback-related settlements Salix Pharmaceuticals, Inc. Olympus Corporation of the Americas Novartis Pharmaceuticals Warner Chilcott Omnicare, Inc. FALSE CLAIM ACT SETTLEMENTS Settlements involving sales and marketing issues Acclarent, Inc. Genentech Inc. and OSI Pharmaceuticals LLC Paradigm Spine Qualitest Pharmaceuticals Settlement involving Medicaid drug rebate issues Wyeth/Pfizer Settlements involving other issues Imported and compounded drugs CIVIL MONETARY PENALTY SETTLEMENTS Drug Price Reporting CMP Settlements Nephron Pharmaceuticals Corporation Cipher Pharmaceuticals US, LLC Coloplast Corp. OIG REPORTS Drug pricing issues “Average Manufacturer Prices Increased Faster Than Inflation for Many Generic Drugs” – December 2015 (A-06-15-00030) 340B program issues “Part B Payment for 340B Purchased Drugs” - Nov. 2015 (OEI-12-14-00030) “State Efforts to Exclude 340B drugs from Medicaid Managed Care Rebates” – June 2016 (OEI-05-14- 00430) OIG REPORTS Reports relating to the Medicaid -
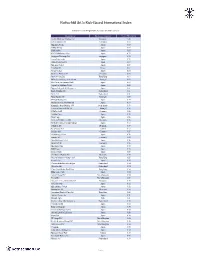
R&Co Risk-Based International Index – Weighting
Rothschild & Co Risk-Based International Index Indicative Index Weight Data as of June 30, 2021 on close Constituent Exchange Country Index Weight(%) Jardine Matheson Holdings Ltd Singapore 1.46 LEG Immobilien SE Germany 0.98 Ajinomoto Co Inc Japan 0.95 SoftBank Corp Japan 0.89 Shimano Inc Japan 0.85 FUJIFILM Holdings Corp Japan 0.73 Singapore Exchange Ltd Singapore 0.72 Japan Tobacco Inc Japan 0.72 Cellnex Telecom SA Spain 0.69 Nintendo Co Ltd Japan 0.69 Carrefour SA France 0.67 Nexon Co Ltd Japan 0.66 Deutsche Wohnen SE Germany 0.65 Bank of China Ltd Hong Kong 0.64 REN - Redes Energeticas Nacion Portugal 0.63 Pan Pacific International Hold Japan 0.63 Japan Post Holdings Co Ltd Japan 0.62 Nippon Telegraph & Telephone C Japan 0.61 Roche Holding AG Switzerland 0.61 Nestle SA Switzerland 0.61 Novo Nordisk A/S Denmark 0.59 ENEOS Holdings Inc Japan 0.59 Nomura Research Institute Ltd Japan 0.59 Koninklijke Ahold Delhaize NV Netherlands 0.59 Jeronimo Martins SGPS SA Portugal 0.58 HelloFresh SE Germany 0.58 Toshiba Corp Japan 0.58 Hoya Corp Japan 0.58 Siemens Healthineers AG Germany 0.58 MS&AD Insurance Group Holdings Japan 0.57 Coloplast A/S Denmark 0.57 Kerry Group PLC Ireland 0.57 Scout24 AG Germany 0.57 SG Holdings Co Ltd Japan 0.56 Symrise AG Germany 0.56 Nitori Holdings Co Ltd Japan 0.56 Beiersdorf AG Germany 0.55 Mitsubishi Corp Japan 0.55 KDDI Corp Japan 0.55 Sysmex Corp Japan 0.55 Chr Hansen Holding A/S Denmark 0.55 Ping An Insurance Group Co of Hong Kong 0.55 Eisai Co Ltd Japan 0.54 Chocoladefabriken Lindt & Spru Switzerland 0.54 Givaudan -

Association Between Variability in Body Mass Index and Development of Type 2 Diabetes: Panasonic Cohort Study
Epidemiology/Health services research Open access Original research BMJ Open Diab Res Care: first published as 10.1136/bmjdrc-2021-002123 on 22 April 2021. Downloaded from Association between variability in body mass index and development of type 2 diabetes: Panasonic cohort study Hiroshi Okada ,1 Masahide Hamaguchi ,2 Momoko Habu,1 Kazushiro Kurogi,3 Hiroaki Murata,4 Masato Ito,3 Michiaki Fukui2 To cite: Okada H, ABSTRACT Hamaguchi M, Habu M, Introduction Contrasting results have been reported for Significance of this study et al. Association between the association between the variability in body weight variability in body mass index and development of diabetes. In the present study, we What is already known about this subject? and development of type 2 evaluated the association between the variability in body ► Obesity or body weight gain accelerate the develop- diabetes: Panasonic cohort ment of diabetes. study. BMJ Open Diab Res Care mass index (BMI) and development of type 2 diabetes in 19 412 Japanese participants without obesity and without ► The association between the variability in body 2021;9:e002123. doi:10.1136/ weight and development of diabetes is controversial. bmjdrc-2021-002123 body weight gain or loss during the study period. Research design and methods We recorded body weight What are the new findings? of the participants consecutively each year in Panasonic ► The risk for developing diabetes increased by 11.1% Received 9 January 2021 Corporation, Osaka, Japan from 2008 to 2014 to evaluate Revised 26 February 2021 per 1% increase in coefficient of variation of body the variability of BMI. The participants with obesity (BMI mass index (BMI). -

OSI Pharmaceuticals and AVEO Pharmaceuticals Enter Into an Oncology Drug Discovery and Translational Research Collaboration | Busi…
3/9/2019 OSI Pharmaceuticals and AVEO Pharmaceuticals Enter into an Oncology Drug Discovery and Translational Research Collaboration | Busi… 111.92.25.247 OSI Pharmaceuticals and AVEO Pharmaceuticals Enter into an Oncology Drug Discovery and Translational Research Collaboration - OSI to Host Conference Call / Webcast - October 02, 2007 07:30 AM Eastern Daylight Time MELVILLE, N.Y. & CAMBRIDGE, Mass.--(BUSINESS WIRE)--OSI Pharmaceuticals, Inc. (Nasdaq: OSIP) and AVEO Pharmaceuticals, Inc. announced today that they have entered into a small molecule drug discovery and translational research collaboration. The alliance is anchored around developing molecular targeted therapies that target the underlying mechanisms of epithelial-mesenchymal transition (EMT) in cancer, a process of emerging significance in tumor development and disease progression and the focal point of OSI’s proprietary oncology research efforts. The companies will collaborate to develop proprietary target-driven tumor models for use in drug screening and biomarker validation, and intend to deploy these systems in support of OSI drug discovery and clinical programs. Under the terms of the agreement, OSI will pay AVEO a total upfront of $10 million in cash and purchase equity valued at approximately $5 million. OSI will also pay AVEO research funding, and milestones and royalties upon successful development and commercialization of products from the collaboration. “We are delighted to announce this collaboration with AVEO,” stated Colin Goddard, Ph.D., Chief Executive Officer -

Notice of the Ordinary General Meeting of Shareholders to Be Held on June 26, 2020
SONY CORPORATION Notice of the Ordinary General Meeting of Shareholders to be held on June 26, 2020 To the Registered Holders of American Depositary Receipts representing shares of Common Stock of Sony Corporation (the “Corporation”): The undersigned Depositary has received a notice that the Corporation has called an ordinary general meeting of shareholders to be held in Tokyo, Japan on June 26, 2020 (the “Meeting”) for the following purposes: MATTERS TO BE REPORTED: To receive reports on the business report, non-consolidated financial statements, consolidated financial statements, as well as audit reports on the consolidated financial statements by the Independent Auditors (certified public accountants) and the Audit Committee for the fiscal year ended March 31, 2020 (from April 1, 2019 to March 31, 2020) pursuant to the Companies Act of Japan. PROPOSALS TO BE ACTED UPON: 1. To amend a part of the Articles of Incorporation. 2. To elect 12 Directors. 3. To issue Stock Acquisition Rights for the purpose of granting stock options. EXPLANATION REGARDING THE SUBJECT MATTER OF THE MEETING MATTERS TO BE REPORTED: To receive reports on the business report, non-consolidated financial statements, consolidated financial statements, as well as audit reports on the consolidated financial statements by the Independent Auditors (certified public accountants) and the Audit Committee for the fiscal year ended March 31, 2020 (from April 1, 2019 to March 31, 2020). Note: The Consolidated Financial Statements pursuant to the Companies Act of Japan (Translation) are available on the Sony Investor Relations website. This document can be accessed at https://www.sony.net/SonyInfo/IR/stock/shareholders_meeting/Meeting103/ 1 PROPOSALS TO BE ACTED UPON: 1. -

Clinical Research Services
Clinical Research Services Advancing the future of medicine through research. Who We Are Experience Clinical Research Services is a department within Clinical Research Services has been involved CHI St. Alexius Health in Bismarck, ND. We are fully in clinical research since 1987. Our professional dedicated to providing research support services staff has more than 180 years of combined to physicians within our region and the valued experience in clinical trials. We are committed patients they serve. to providing outstanding service to ensure the success of every project. CHI St. Alexius Health is a tertiary care facility affiliated with PrimeCare, a health group which Our involvement in inpatient and outpatient combines the most experienced, trusted, and phase II-IV clinical and device research studies has proven medical leaders in the area. PrimeCare is contributed substantially to the approval of new comprised of a network which includes more than drugs and treatments. You should carefully consider 190 physicians in private and institutional practice, both the benefits and the risks of participation including: CHI St. Alexius Health, Mid Dakota Clinic, before enrolling in a study. The Bone & Joint Center, and other affiliated area physicians. Many of these physicians are actively Previous clinical study trials: involved as investigators for clinical trials. • Oncology • Orthopaedic • Rheumatology • Diabetes Our clinical research team is committed to providing: • Neurology • Weight loss • Rapid study start-up • Cardiology • Pain • Pro-active patient enrollment • Urology • Men’s Health • Clean data submission • Gastroenterology • Women’s Health • Highly experienced principal • Infectious Disease • Medical Devices investigators • Pediatric • Initial training and continuing education for all support personnel Sponsors Patient Demographics Abbott Laboratories Characteristics of our patients include Acorda Therapeutics, Inc.