IPR2016-01284 Patent 6,900,221 B1 ______
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

OSI Pharmaceuticals and AVEO Pharmaceuticals Expand Oncology Discovery and Translational Research Collaboration
OSI Pharmaceuticals and AVEO Pharmaceuticals Expand Oncology Discovery and Translational Research Collaboration July 21, 2009 9:24 PM ET MELVILLE, NY and CAMBRIDGE, Mass., July 21, 2009 – OSI Pharmaceuticals, Inc. (NASDAQ:OSIP) and AVEO Pharmaceuticals, Inc. today announced that they have expanded the drug discovery and translational research collaboration announced in October of 2007. The alliance is anchored around developing molecular targeted therapies to target the underlying mechanisms of epithelial-mesenchymal transition (EMT) in cancer and to develop proprietary datasets of associated patient selection biomarkers to support OSI’s targeted medicine pipeline. EMT is a process of emerging significance in tumor development and disease progression and the focal point of OSI’s proprietary oncology research efforts. The companies are expanding their efforts to validate cancer targets and to deploy key elements of AVEO’s proprietary translational research platform in support of OSI’s clinical development programs. Under the terms of the expanded discovery and research agreement, OSI will pay AVEO a total of $20 million at closing, $5 million of which is an upfront cash payment and $15 million of which is the purchase of additional equity in AVEO. OSI will also provide AVEO research funding over the next two years to support the collaboration, and the potential to achieve additional royalties and milestones. In return, OSI will immediately receive rights beyond the original collaboration including rights to additional EMT targets (including up to 4 antibody targets) and increased access to AVEO technology (i.e. tumor models, archives and biomarkers). OSI is also acquiring non-exclusive access to AVEO’s proprietary bioinformatics platform. -

In the United States District Court for the District of Delaware
Case 1:15-cv-01063-UNA Document 1 Filed 11/17/15 Page 1 of 17 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE OSI PHARMACEUTICALS, LLC, PFIZER ) INC., and GENENTECH, INC., ) ) Plaintiffs, ) ) v. ) C.A. No. ) BRECKENRIDGE PHARMACEUTICAL, ) INC. and NATCO PHARMA LTD., ) ) Defendants. ) COMPLAINT FOR PATENT INFRINGEMENT Plaintiffs OSI Pharmaceuticals, LLC (“OSI”), Pfizer Inc. (“Pfizer”) and Genentech, Inc. (“Genentech”) (collectively, “Plaintiffs”), by their undersigned attorneys, bring this action against Defendant Breckenridge Pharmaceutical Inc., (“Breckenridge”), and Natco Pharma Ltd. (“Natco”) (collectively, “Breckenridge/Natco”) for patent infringement and allege as follows: NATURE OF THE ACTION 1. This is an action for patent infringement under the patent laws of the United States, Title 35 of the United States Code, the Federal Food, Drug, and Cosmetic Act, Title 21 of the United States Code, and the Declaratory Judgment Act, 28 U.S.C. §§ 2201 et seq., arising from Breckenridge/Natco’s filing of an Abbreviated New Drug Application No. 208488 (“Breckenridge/Natco ANDA” or “ANDA No. 208488”) with the United States Food and Drug Administration (“FDA”) seeking approval to commercially market generic versions of TARCEVA® (“Breckenridge/Natco’s Proposed Generic Products”) prior to the expiration of certain patents that relate to that product, United States Reissued Patent No. RE 41,065 (“the RE ‘065 patent”), and United States Patent No. 6,900,221 (“the ‘221 patent”). Case 1:15-cv-01063-UNA Document 1 Filed 11/17/15 Page 2 of 17 PageID #: 2 THE PARTIES 2. Plaintiff OSI is a limited liability company organized and existing under the laws of the State of Delaware, with a principal place of business at 1 Astellas Way, Northbrook, IL 60062. -

17TH ANNUAL PHARMACEUTICAL and MEDICAL DEVICE COMPLIANCE CONGRESS Office of Inspector General Update
17TH ANNUAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS Office of Inspector General Update Mary E. Riordan, Senior Counsel Office of Counsel to the Inspector General October 19, 2016 AGENDA FOR TODAY Update on recent enforcement activity Update on OIG reports and other publications Lessons/suggestions for consideration ISSUES IN RECENT SETTLEMENTS Settlements addressed a variety of areas: Kickback issues Marketing and sales issues Medicaid drug rebate program issues Drug price reporting issues FALSE CLAIM ACT SETTLEMENTS Kickback-related settlements Salix Pharmaceuticals, Inc. Olympus Corporation of the Americas Novartis Pharmaceuticals Warner Chilcott Omnicare, Inc. FALSE CLAIM ACT SETTLEMENTS Settlements involving sales and marketing issues Acclarent, Inc. Genentech Inc. and OSI Pharmaceuticals LLC Paradigm Spine Qualitest Pharmaceuticals Settlement involving Medicaid drug rebate issues Wyeth/Pfizer Settlements involving other issues Imported and compounded drugs CIVIL MONETARY PENALTY SETTLEMENTS Drug Price Reporting CMP Settlements Nephron Pharmaceuticals Corporation Cipher Pharmaceuticals US, LLC Coloplast Corp. OIG REPORTS Drug pricing issues “Average Manufacturer Prices Increased Faster Than Inflation for Many Generic Drugs” – December 2015 (A-06-15-00030) 340B program issues “Part B Payment for 340B Purchased Drugs” - Nov. 2015 (OEI-12-14-00030) “State Efforts to Exclude 340B drugs from Medicaid Managed Care Rebates” – June 2016 (OEI-05-14- 00430) OIG REPORTS Reports relating to the Medicaid -

OSI Pharmaceuticals and AVEO Pharmaceuticals Enter Into an Oncology Drug Discovery and Translational Research Collaboration | Busi…
3/9/2019 OSI Pharmaceuticals and AVEO Pharmaceuticals Enter into an Oncology Drug Discovery and Translational Research Collaboration | Busi… 111.92.25.247 OSI Pharmaceuticals and AVEO Pharmaceuticals Enter into an Oncology Drug Discovery and Translational Research Collaboration - OSI to Host Conference Call / Webcast - October 02, 2007 07:30 AM Eastern Daylight Time MELVILLE, N.Y. & CAMBRIDGE, Mass.--(BUSINESS WIRE)--OSI Pharmaceuticals, Inc. (Nasdaq: OSIP) and AVEO Pharmaceuticals, Inc. announced today that they have entered into a small molecule drug discovery and translational research collaboration. The alliance is anchored around developing molecular targeted therapies that target the underlying mechanisms of epithelial-mesenchymal transition (EMT) in cancer, a process of emerging significance in tumor development and disease progression and the focal point of OSI’s proprietary oncology research efforts. The companies will collaborate to develop proprietary target-driven tumor models for use in drug screening and biomarker validation, and intend to deploy these systems in support of OSI drug discovery and clinical programs. Under the terms of the agreement, OSI will pay AVEO a total upfront of $10 million in cash and purchase equity valued at approximately $5 million. OSI will also pay AVEO research funding, and milestones and royalties upon successful development and commercialization of products from the collaboration. “We are delighted to announce this collaboration with AVEO,” stated Colin Goddard, Ph.D., Chief Executive Officer -

Clinical Research Services
Clinical Research Services Advancing the future of medicine through research. Who We Are Experience Clinical Research Services is a department within Clinical Research Services has been involved CHI St. Alexius Health in Bismarck, ND. We are fully in clinical research since 1987. Our professional dedicated to providing research support services staff has more than 180 years of combined to physicians within our region and the valued experience in clinical trials. We are committed patients they serve. to providing outstanding service to ensure the success of every project. CHI St. Alexius Health is a tertiary care facility affiliated with PrimeCare, a health group which Our involvement in inpatient and outpatient combines the most experienced, trusted, and phase II-IV clinical and device research studies has proven medical leaders in the area. PrimeCare is contributed substantially to the approval of new comprised of a network which includes more than drugs and treatments. You should carefully consider 190 physicians in private and institutional practice, both the benefits and the risks of participation including: CHI St. Alexius Health, Mid Dakota Clinic, before enrolling in a study. The Bone & Joint Center, and other affiliated area physicians. Many of these physicians are actively Previous clinical study trials: involved as investigators for clinical trials. • Oncology • Orthopaedic • Rheumatology • Diabetes Our clinical research team is committed to providing: • Neurology • Weight loss • Rapid study start-up • Cardiology • Pain • Pro-active patient enrollment • Urology • Men’s Health • Clean data submission • Gastroenterology • Women’s Health • Highly experienced principal • Infectious Disease • Medical Devices investigators • Pediatric • Initial training and continuing education for all support personnel Sponsors Patient Demographics Abbott Laboratories Characteristics of our patients include Acorda Therapeutics, Inc. -

Acquisition of OSI Pharmaceuticals, Inc
Acquisition of OSI Pharmaceuticals, Inc. -Becoming a Global Category Leader in Oncology- May 17, 2010 Table of Contents I. Transaction Summary II. Strategic Rationale III. Overview of OSI Pharmaceuticals IV. Financial Impact 2 1 I. Transaction Summary 3 2 Transaction Summary ● Purchase price: $57.50 per share in cash (55% premium to the closing price on February 26, 2010, the last trading day before the announcement of tender offer) ● Acquisition amount: Approximately $4.0 Billion (Fully diluted basis) ● Tender offer period: Expires no later than 10 business days after the amendment to the Schedule TO is filed (which is expected to be filed on or before May 21st), unless extended ● Financing: Fully financed with cash and cash equivalents on Astellas’ balance sheet ● The acquisition is unanimously approved by the Board of Directors of both Astellas and OSI 4 3 II. Strategic Rationale 5 4 Business Model of Astellas GGloballobal CCategoryategory LLeadereader (GCL)(GCL) A “GCL”, by providing value-added products “globally”, takes over the position of “leader” in a “category” where high unmet medical needs exist and a high degree of expertise is required. 6 5 Becoming a GCL in Oncology ● Oncology, one of Astellas’ five focus therapeutic areas ● Acquisition of OSI significantly enhances Astellas’ oncology capabilities Current Transplantation Urology GCL Areas Immunology & Oncology Infectious Diseases Future GCL Candidates DM Complications & Neuroscience Metabolic Diseases 7 Strategic Rationale for OSI Acquisition ● Accelerates development of -

Manufacturers/Wholesalers Street City ST Zip 454 Life Sciences Branford CT A.L.I Holdings, LLC A.L.I
Manufacturers/Wholesalers Street City ST Zip 454 Life Sciences Branford CT A.L.I Holdings, LLC A.L.I. Imaging Systems Corp A.L.I. Technologies (International) LLC Abbott Diabetes Care Division Abbott Diagnostic Division Abbott Laboratories 100 Abbott Park Rd. Bldg. AP6D-1 Abbott Park IL 60064 Abbott Molecular Division Abbott Nutrition Products Division Abbott Pharmaceutical Products Group Abbott Point of Care Division Abbott Vascular Division Abraxis Bioscience, LLC. 11755 Wilshire Blvd., Suite 2000 Los Angeles CA 90025 Access Diabetic Supply, LLC Acclarent, Inc. 1525-B O'Brien Dr. Menlo Park CA 94025 Acorda Therapeutics, Inc. 15 Skyline Drive Hawthorne NY 10532 Advanced Neuromodulation Systems, Inc. Advanced Respiratory, Inc. Advanced Sterilization Products 33 Technology Drive Irvine CA 92618 Aesculap Implant Systems, Inc. 3773 Corporate Parkway Center Valley PA 18034 Aesculap, Inc. 3773 Corporate Parkway Center Valley PA 18034 Alaven Pharmaceuticals, LLC 2260 Northwest parkway Suite A Marietta GA 30067 Alcon Laboratories, Inc. 6201 South Freeway Fort Worth TX 76134 Alexion Pharmaceuticals, Inc. 352 Knotter Drive Cheshire CT 06410 Alkermes, Inc. 88 Sidney Street Cambridge MA 02139 Allen Medical Systems, Inc. Allergan Sales, LLC Allergan USA, Inc. Allergan, Inc. 2525 Dupont Drive Irvine CA 92612 Alpharma Pharmaceuticals, Inc. Alpharma Pharmaceuticals, LLC AMAG Pharmaceuticals, Inc. 100 Hayden Avenue Lexington MA 02421 AMATECH Corporation American Medical Distributors, Inc. 100 New Highway N Amityville NY 11701 American Medical System, -

Healthcare Conference
25th Annual HEALTHCARE CONFERENCE Healthcare Westin St. Francis San Francisco January 8–11, 2007 JPMorgan cordially invites you to attend the 25th Annual Healthcare Conference, January 8 –11, 2007, at the Westin St. Francis in San Francisco. JPMorgan’s premier conference on the healthcare industry will feature more than 260 public and private companies over four days of simultaneous sessions. In addition, the conference will host topical panel discussions featuring leading industry experts. Preliminary List of Invited Presenting Companies Actelion Ltd Baxter International Inc. Cooper Companies Aetna Incorporated BD (Becton, Dickinson) Coventry Affymetrix, Inc. Beckman Coulter, Inc. Dade Behring Holdings, Inc. Alcon, Inc. Bioenvision, Inc. DaVita Inc. Align Technology, Inc. Biogen Idec deCODE genetics, Inc. Allscripts Healthcare Solutions Inc. Biomet, Inc. Digene Altana Boston Scientific Corporation Digirad Corporation — US American Medical Systems Holdings Inc. Bristol-Myers Squibb Company Diversa Corporation Amerigroup Corp. Cambrex Corporation DJO Incorporated AmerisourceBergen Cardinal Health, Inc. Dyax Corp. Amgen, Inc. Caremark Rx, Inc. Eclipsys Corporation AMN Healthcare Services, Inc. Celgene Corporation Edwards Lifesciences Corporation AmSurg Corporation Cell Genesys Elan Corporation, plc Anadys Pharmaceuticals CENTENE Corporation Eli Lilly and Company Applied Biosystems Group Cephalon, Inc. Emdeon Ariad Pharmaceuticals, Inc. Cerner Corporation Emergency Medical Services Corporation AstraZeneca Group plc Charles River Laboratories, -
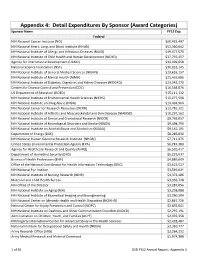
Appendix 4: Detail Expenditures by Sponsor (Award Categories) Sponsor Name FY12 Exp
Appendix 4: Detail Expenditures By Sponsor (Award Categories) Sponsor Name FY12 Exp. Federal NIH National Cancer Institute (NCI) $60,455,497 NIH National Heart, Lung, and Blood Institute (NHLBI) $52,360,642 NIH National Institute of Allergy and Infectious Diseases (NIAID) $49,477,570 NIH National Institute of Child Health and Human Development (NICHD) $37,797,457 Agency for International Development (USAID) $34,499,658 National Science Foundation (NSF) $30,033,145 NIH National Institute of General Medical Sciences (NIGMS) $29,655,157 NIH National Institute of Mental Health (NIMH) $25,435,686 NIH National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDKD) $23,042,270 Centers for Disease Control and Prevention (CDC) $16,568,876 US Department of Education (DOED) $15,111,132 NIH National Institute of Environmental Health Sciences (NIEHS) $15,077,598 NIH National Institute on Drug Abuse (NIDA) $14,494,964 NIH National Center for Research Resources (NCRR) $12,781,321 NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMSD) $10,297,162 NIH National Institute of Dental and Craniofacial Research (NIDCR) $9,769,057 NIH National Institute of Neurological Disorders and Stroke (NINDS) $9,498,790 NIH National Institute on Alcohol Abuse and Alcoholism (NIAAA) $9,161,191 Department of Energy (DOE) $8,285,830 NIH National Human Genome Research Institute (NHGRI) $7,711,975 United States Environmental Protection Agency (EPA) $6,787,380 Agency for Healthcare Research and Quality (AHRQ) $6,520,417 Department of Homeland -

Astellas Goes Hostile Again, OSI the Target
March 01, 2010 Astellas goes hostile again, OSI the target Evaluate Vantage One year on from Astellas Pharma’s failed $1bn bid for CV Therapeutics, the hope must be the Japanese company has since been brushing up its hostile takeover skills with news today that, having failed to convince OSI Pharmaceuticals’ board of directors of the value and merit of a tie-up, Astellas is going straight to OSI’s shareholders with a $52 cash per share offer, valuing the company at $3.5bn. Not content with being one of the most aggressive global pharma groups on the product licensing front last year, Astellas clearly still craves a major acquisition to address its pipeline and patent cliff woes. As such, its move on OSI is hardly surprising (Astellas walks away from CV but hunt for deals likely to continue, March 16, 2009). OSI’s shares opened at $55.45, a 50% gain and a price its shareholders have not seen for five years, suggesting the market is hopeful that either Astellas will have to increase its 40% premium offer, or that a white knight may swoop, as Gilead Sciences did for CV. OSI has yet to formally respond to Astellas’ offer, although anything other than a standard riposte that it “very significantly undervalues” OSI, as the board previously told Astellas, would be a major surprise. Tempting offer? Astellas will launch its offer tomorrow, and has sufficient cash in the bank - $4.2bn as of December 31, 2009 - to do so without the need for additional finance. The question now for OSI’s shareholders is whether to jump at this opportunity or believe its own board that even greater value could be extracted in the future. -

Advanced Sterilization Products Alpharma Pharmaceuticals
Manufacturers/Wholesalers Street City ST Zip Abbott Diabetes Care Abbott Diagnostic Division Abbott Laboratories 100 Abbott Park Rd. Bldg. AP6D-1 Abbott Park IL 60064 Abbott Molecular Abbott Nutrition Abbott Pharmaceutical Products Group Abbott Point of Care Abbott Spine Abbott Vascular Abraxis Bioscience, LLC. 11755 Wilshire Blvd., Suite 2000 Los Angeles CA 90025 Access Diabetic Supply, LLC Acorda Therapeutics, Inc. 15 Skyline Drive Hawthorne NY 10532 Actelion Pharmaceuticals US, Inc. 5000 Shoreline Court, Suite 200 S. San Francisco CA 94080 Advanced Medical Optics, Inc. 1700 E. St. Andrew Place Santa Anna CA 92705 Advanced Neuromodulation Systems, Inc. Advanced Sterilization Products 33 Technology Drive Irvine CA 92618 Agencourt Bioscience Corporation Alcon Laboratories, Inc. 6201 South Freeway Fort Worth TX 76134 Alexion Pharmaceuticals, Inc. 352 Knotter Drive Cheshire CT 06410 Alkermes, Inc. 88 Sidney Street Cambridge MA 02139 Allergan American, LLC. Allergan Holdings, Inc, Allergan Puerto Rico Holdings, Inc. Allergan Sales Allergan Sales Puerto Rico, Inc. Allergan Specialty Therapeutics, Inc. Allergan USA, Inc. Allergan, Inc. 2525 Dupont Drive Irvine CA 92612 Allscripts, LLC. 2401 Commerce Drive Libertyville IL 60048 Alpharma Pharmaceuticals, LLC 1 New England Ave Piscataway NJ 08854 American Medical System, Inc 10700 Bren Road West Minnetonka MN 55343 Ameridose, LLC. 50 Fountain Street Framingham MA 01702 Amgen, Inc. One Amgen Center Drive Thousand Oaks CA 91320 Amgen, USA AMO Sales and Service, Inc. Amphastar Pharmaceuticals, Inc. 11570 Sixth St. Rancho Cucamnga CA 91730 AMS- AMS Innovation Center 3070 Orchard Drive San Jose CA 95134 AMS Sales Corporation 10700 Bren Road West Minnetonka MN 55343 Amylin Pharmaceutical, Inc. 9360 Towne Center Drive San Diego CA 92121 Anda Pharmaceuticals, Inc. -

11MAR201513234945 1FEB201511060799 777 Old Saw Mill River Road Tarrytown, New York 10591-6707
11MAR201513234945 1FEB201511060799 777 Old Saw Mill River Road Tarrytown, New York 10591-6707 April 21, 2015 Dear Fellow Shareholders: It is my pleasure to invite you to attend the 2015 Annual Meeting of Shareholders of Regeneron Pharmaceuticals, Inc. to be held on Friday, June 12, 2015 at 10:30 a.m., Eastern Time, at the Westchester Marriott Hotel, 670 White Plains Road, Tarrytown, New York 10591. This year we are again using the ‘‘Notice and Access’’ method of providing proxy materials to you via the Internet. We believe that this process provides you with a convenient and quick way to access your proxy materials and vote your shares, while allowing us to reduce the costs of printing and distributing the proxy materials and conserve resources. On or about April 27, 2015, we will mail to our shareholders a Notice of Internet Availability of Proxy Materials containing instructions on how to access our proxy statement and our 2014 annual report and vote via the Internet. The Notice also contains instructions on how to receive a paper copy of the proxy materials and our 2014 annual report. Similar to last year, our proxy materials are presented in an enhanced format, with a Proxy Summary and an expanded Compensation Discussion and Analysis, which we hope will make your review of these materials easier. One of the priorities of the board of directors and Company management is ensuring robust outreach and engagement with our shareholders. Over the last year, we have spent a significant amount of time speaking with some of our shareholders about executive compensation and corporate governance.