Website Fatigue, Shortness of Breath, Weight Loss, and Poor Appetite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Hygiene Plan Manual
CHEMICAL HYGIENE PLAN AND HAZARDOUS MATERIALS SAFETY MANUAL FOR LABORATORIES This is the Chemical Hygiene Plan specific to the following areas: Laboratory name or room number(s): ___________________________________ Building: __________________________________________________________ Supervisor: _______________________________________________________ Department: _______________________________________________________ Telephone numbers 911 for Emergency and urgent consultation 48221 Police business line 46919 Fire Dept business line 46371 Radiological and Environmental Management Revisied on: Enter a revision date here. All laboratory chemical use areas must maintain a work-area specific Chemical Hygiene Plan which conforms to the requirements of the OSHA Laboraotry Standard 29 CFR 19190.1450. Purdue University laboratories may use this document as a starting point for creating their work area specific CHP. Minimally this cover page is to be edited for work area specificity (non-West Lafayette laboratories are to place their own emergency, fire, and police telephone numbers in the space above) AND appendix K must be completed. This instruction and information box should remain. This model CHP is version 2010A; updates are to be found at www.purdue.edu/rem This page intentionally blank. PURDUE CHEMICAL HYGIENE PLAN AWARENESS CERTIFICATION For CHP of: ______________________________ Professor, building, rooms The Occupational Safety and Health Administration (OSHA) requires that laboratory employees be made aware of the Chemical Hygiene Plan at their place of employment (29 CFR 1910.1450). The Purdue University Chemical Hygiene Plan and Hazardous Materials Safety Manual serves as the written Chemical Hygiene Plan (CHP) for laboratories using chemicals at Purdue University. The CHP is a regular, continuing effort, not a standby or short term activity. Departments, divisions, sections, or other work units engaged in laboratory work whose hazards are not sufficiently covered in this written manual must customize it by adding their own sections as appropriate (e.g. -

Toxicological Profile for Beryllium
BERYLLIUM 19 3. HEALTH EFFECTS 3.1 INTRODUCTION The primary purpose of this chapter is to provide public health officials, physicians, toxicologists, and other interested individuals and groups with an overall perspective on the toxicology of beryllium. It contains descriptions and evaluations of toxicological studies and epidemiological investigations and provides conclusions, where possible, on the relevance of toxicity and toxicokinetic data to public health. A glossary and list of acronyms, abbreviations, and symbols can be found at the end of this profile. 3.2 DISCUSSION OF HEALTH EFFECTS BY ROUTE OF EXPOSURE To help public health professionals and others address the needs of persons living or working near hazardous waste sites, the information in this section is organized first by route of exposure (inhalation, oral, and dermal) and then by health effect (death, systemic, immunological, neurological, reproductive, developmental, genotoxic, and carcinogenic effects). These data are discussed in terms of three exposure periods: acute (14 days or less), intermediate (15–364 days), and chronic (365 days or more). Levels of significant exposure for each route and duration are presented in tables and illustrated in figures. The points in the figures showing no-observed-adverse-effect levels (NOAELs) or lowest-observed-adverse-effect levels (LOAELs) reflect the actual doses (levels of exposure) used in the studies. LOAELS have been classified into "less serious" or "serious" effects. "Serious" effects are those that evoke failure in a biological system and can lead to morbidity or mortality (e.g., acute respiratory distress or death). "Less serious" effects are those that are not expected to cause significant dysfunction or death, or those whose significance to the organism is not entirely clear. -

Fluoride Inhibitor (BS1077-B) Ghsunitedstatessds En 2021-02-01
Page: 1/9 Safety Data Sheet according to OSHA HCS (29CFR 1910.1200) and WHMIS 2015 Regulations Revision: January 27, 2021 1 Identification · Product identifier · Trade name: Fluoride Inhibitor · Product code: BS1077-B · Recommended use and restriction on use · Recommended use: Laboratory chemicals · Restrictions on use: No relevant information available. · Details of the supplier of the Safety Data Sheet · Manufacturer/Supplier: AquaPhoenix Scientific, Inc. 860 Gitts Run Road Hanover, PA 17331 USA Tel +1 (717)632-1291 Toll-Free: (866)632-1291 [email protected] · Distributor: AquaPhoenix Scientific 860 Gitts Run Road, Hanover, PA 17331 (717) 632-1291 · Emergency telephone number: ChemTel Inc. (800)255-3924 (North America) +1 (813)248-0585 (International) 2 Hazard(s) identification · Classification of the substance or mixture Skin Sens. 1 H317 May cause an allergic skin reaction. Carc. 1B H350 May cause cancer. STOT RE 1 H372 Causes damage to organs through prolonged or repeated exposure. · Label elements · GHS label elements The product is classified and labeled according to the Globally Harmonized System (GHS). · Hazard pictograms: GHS07 GHS08 · Signal word: Danger · Hazard statements: H317 May cause an allergic skin reaction. H350 May cause cancer. H372 Causes damage to organs through prolonged or repeated exposure. · Precautionary statements: P201 Obtain special instructions before use. P202 Do not handle until all safety precautions have been read and understood. (Cont'd. on page 2) 50.1.3 Page: 2/9 Safety Data Sheet according to OSHA HCS (29CFR 1910.1200) and WHMIS 2015 Regulations Revision: January 27, 2021 Trade name: Fluoride Inhibitor (Cont'd. of page 1) P260 Do not breathe dust/fume/gas/mist/vapors/spray. -

Beryllium and Beryllium Compounds
BERYLLIUM AND BERYLLIUM COMPOUNDS Beryllium and beryllium compounds were considered by previous IARC Working Groups in 1971, 1979, 1987, and 1993 (IARC, 1972, 1980, 1987, 1993). Since that time, new data have become available, these have been incorporated in the Monograph, and taken into consid- eration in the present evaluation. 1. Exposure Data a very high strength-to-weight ratio. Beryllium is lighter than aluminium but is greater than 40% 1.1 Identification of the agents more rigid than steel. It has excellent electrical and thermal conductivities. Its only markedly Synonyms and molecular formulae for beryl- adverse feature is relatively pronounced brittle- lium, beryllium–aluminium and beryllium– ness, which restricts the use of metallic beryl- copper alloys, and certain beryllium compounds lium to specialized applications (WHO, 1990). are presented in Table 1.1. The list is not exhaus- Because of its low atomic number, beryllium tive, nor does it comprise necessarily the most is very permeable to X-rays. Neutron emission commercially important beryllium-containing after bombardment with α or γ rays is the most substances; rather, it indicates the range of beryl- important of its nuclear physical properties, lium compounds available. and beryllium can be used as a neutron source. Moreover, its low neutron absorptiveness and high-scattering cross-section make it a suitable 1.2 Chemical and physical properties moderator and reflector in structural materials of the agents in nuclear facilities; where most other metals absorb neutrons emitted during the fission Beryllium (atomic number, 4; relative atomic of nuclear fuel, beryllium atoms only reduce mass, 9.01) is a metal, which belongs to Group the energy of such neutrons, and reflect them IIA of the Periodic Table. -

Beryllium CAS #: 7440-41-7 Revised By: RRD Toxicology Unit Revision Date: August 14, 2015
CHEMICAL UPDATE WORKSHEET Chemical Name: Beryllium CAS #: 7440-41-7 Revised By: RRD Toxicology Unit Revision Date: August 14, 2015 (A) Chemical-Physical Properties Part 201 Value Updated Value Reference Source Comments Molecular Weight (g/mol) 9.012 9.01 EPI EXP Physical State at ambient temp Inorganic Inorganic MDEQ Melting Point (˚C) --- 986 PP EXP Boiling Point (˚C) 2471 2468 HSDB EXP Solubility (ug/L) NA NA NA NA Vapor Pressure (mmHg at 25˚C) NA NR NA NA HLC (atm-m³/mol at 25˚C) NR NR NA NA Log Kow (log P; octanol-water) NR NR NA NA Koc (organic carbon; L/Kg) NR NR NA NA Ionizing Koc (L/kg) NR NA NA Diffusivity in Air (Di; cm2/s) NR NR NA NA Diffusivity in Water (Dw; cm2/s) NR NR NA NA Soil Water Partition Coefficient 790 7.9E+02 SSG EST (Kd; inorganics) CHEMICAL UPDATE WORKSHEET Beryllium (7440-41-7) Part 201 Value Updated Value Reference Source Comments Flash Point (˚C) NA NA NA NA Lower Explosivity Level (LEL; NA NA NA NA unit less) Critical Temperature (K) NR NA NA Enthalpy of Vaporization NR NA NA (cal/mol) Density (g/mL, g/cm3) NR NA NA EMSOFT Flux Residential 2 m NA NR EMSOFT NA (mg/day/cm2) EMSOFT Flux Residential 5 m NA NR EMSOFT NA (mg/day/cm2) EMSOFT Flux Nonresidential 2 m NA NR EMSOFT NA (mg/day/cm2) EMSOFT Flux Nonresidential 5 m NA NR EMSOFT NA (mg/day/cm2) 2 CHEMICAL UPDATE WORKSHEET Beryllium (7440-41-7) (B) Toxicity Values/Benchmarks Source*/Reference Comments/Notes Part 201 Value Updated Value /Date /Issues Reference Dose 1.5E-3 2.0E-3 ATSDR, 2002 (RfD) (mg/kg/day) Dog chronic oral Tier 2 Source: bioassay, BMD10 = ATSDR: Complete 0.46 mg/kg-d; Basis: ATSDR and IRIS use the same critical study; however, ATSDR is more current UF=300; Critical than IRIS. -

Attachment 3-1 Guidance for Developing Ecological Soil
Attachment 3-1 Guidance for Developing Ecological Soil Screening Levels (Eco-SSLs) Eco-SSL Standard Operating Procedure (SOP # 1): Plant and Soil Invertebrate Literature Search and Acquisition OSWER Directive 92857-55 November 2003 This page intentionally left blank OVERVIEW Currently, there is a lack of clear guidance in setting terrestrial effect thresholds when conducting risk assessments. Without an EPA-approved, peer-reviewed, ecologically-based terrestrial effect database, the process to develop thresholds is problematic both to EPA, other federal agencies, states, and concerned private parties. Identification of published toxicity studies on invertebrates, microbial processes and plants is a key step in the derivation of benchmarks. The purpose of the Task Group 4, Standard Operating Procedure Number 1: Literature Search and Acquisition (referred to as TG4-SOP#1) is to document procedures used to identify and acquire potentially relevant toxicology literature for use in setting ecological soil screening levels. The literature search strategy is designed to locate worldwide terrestrial toxicity literature that includes the effects of chemicals of concern on terrestrial soil-dwelling invertebrates and plants. The literature acquisition process is designed to ensure timely acquisition of relevant publications. LITERATURE IDENTIFICATION Potentially relevant literature for developing ecological soil screening levels (Eco-SSLs) is identified by examining hard copies of relevant journals, bibliographies and guidance publications and through the use of a comprehensive computerized literature search strategy. These procedures are designed to locate worldwide terrestrial toxicology literature that includes the effects of specific toxic substances with an emphasis on exposure via soil. Paper-based Literature Identification The paper-based literature identification process includes the scanning of relevant review article bibliographies and key journals held in the U.S. -

Safety Data Sheet
SAFETY DATA SHEET SECTION 1: CHEMICAL PRODUCT and COMPANY IDENTIFICATION Product Name: Beryllium sulfate tetrahydrate 99.9% Product Code: B10160 Supplier: Pfaltz & Bauer, Inc. 172 E. Aurora Street Waterbury, CT 06708 USA Phone: 203-574-0075 FAX: 203-574-3181 Emergency Phone: INFOTRAC, US: 1-800-535-5053 INFOTRAC, INTERNATIONAL: +1-352-323-3500 SECTION 2: HAZARDS IDENTIFICATION Statement of Hazard: Environmentally hazardous, Irritant, Respiratory irritant, Skin sensitizer, Toxic Acute Health Hazard: Irritant to eyes, skin, mucous membranes and respiratory system. May be toxic by ingestion, harmful by skin absorption, and fatal by inhalation. Chronic Health Hazard: Carcinogen, Target organ effect HMIS Rating: H: 3 F: 0 P: 0 NFPA Rating: H: 4 F: 0 R: 0 To the best of our knowledge, the toxicological properties of this chemical have not been thoroughly investigated. Use appropriate procedures and precautions to prevent or minimize exposure. GHS Classification in accordance with 29 CFR 1910 (OSHA HCS): Page 1 of 7 Acute toxicity, dermal (Category 4), H312 Acute toxicity, inhalation (Category 1), H330 Acute toxicity, oral (Category 3), H301 Carcinogenicity (Category 1A), H350 Hazardous to the aquatic environment, chronic toxicity (Category 2), H411 Sensitization, skin (Category 1), H317 Serious eye damage/eye irritation (Category 2A), H319 Skin corrosion/irritation (Category 2), H315 Specific target organ toxicity, repeated exposure (Category 1), H372 Specific target organ toxicity, single exposure; Respiratory tract irritation (Category 3), H335 Pictogram: Signal Word: Danger Hazard Statement(s): H301 Toxic if swallowed. H312 Harmful in contact with skin. H315 Causes skin irritation. H317 May cause an allergic skin reaction. H319 Causes serious eye irritation. -

SDS US 2073 Version #: 05 Revision Date: 03-17-2021 Issue Date: 07-07-2016 1 / 10 Precautionary Statement Prevention Obtain Special Instructions Before Use
SAFETY DATA SHEET 1. Identification Product identifier Beryllium Sulfate Tetrahydrate Other means of identification SDS number M25 CAS number 7787-56-6 Synonyms Beryllium Sulphate Tetrahydrate Manufacturer/Importer/Supplier/Distributor information Manufacturer Company name Materion Brush Inc. Address 6070 Parkland Boulevard Mayfield Heights, OH 44124 United States Telephone 1.800.862.4118 Website www.materion.com E-mail [email protected] Contact person Theodore Knudson Emergency phone number 1.800.862.4118 2. Hazard(s) identification Physical hazards Not classified. Health hazards Acute toxicity, oral Category 3 Acute toxicity, inhalation Category 1 Skin corrosion/irritation Category 2 Serious eye damage/eye irritation Category 2A Sensitization, skin Category 1 Carcinogenicity Category 1 Specific target organ toxicity, single exposure Category 1 Specific target organ toxicity, repeated Category 1 (Respiratory system) exposure Environmental hazards Not classified. OSHA defined hazards Not classified. Label elements Signal word Danger Hazard statement Toxic if swallowed. Fatal if inhaled. Causes severe skin burns and eye damage. Causes serious eye irritation. May cause an allergic skin reaction. May cause cancer. May cause respiratory irritation. Causes damage to organs (respiratory system) through prolonged or repeated exposure by inhalation. Material name: Beryllium Sulfate Tetrahydrate SDS US 2073 Version #: 05 Revision date: 03-17-2021 Issue date: 07-07-2016 1 / 10 Precautionary statement Prevention Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Do not breathe dust. Use only outdoors or in a well-ventilated area. Contaminated work clothing must not be allowed out of the workplace. Avoid release to the environment. Wear protective gloves/protective clothing/eye protection/face protection. -
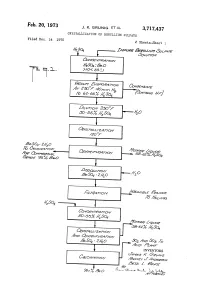
US3717437.Pdf
3,717,437 United States Patent Office Patented Feb. 20, 1973 1 2 3,717,437 pure aqueous sulfuric acid solution containing dissolved CRYSTALLIZATION OF BERYLLUM SULFATE beryllium sulfate which comprises first treating the solu James K. Grunig, Rodney J. Anderson, and Bess L. Vance, tion to form a solution containing about 50% to 55% Tucson, Ariz, assignors to The Anaconda Company by weight of sulfuric acid. The treated solution is then Filed Dec. 14, 1970, Ser. No. 97,841 cooled to a temperature sufficiently low to crystallize Int. Cl, C22b 59/00 beryllium sulfate therefrom and produce an acid mother U.S. CI. 23-24 B 16 Claims liquor containing about 58% to 62% by weight Sulfuric acid. The acidic mother liquor is thereafter separated from the beryllium sulfate crystals and the beryllium sulfate ABSTRACT OF THE DISCLOSURE O crystals are calcined to form beryllium oxide of suf A process for the recovery of substantially pure beryl ficiently high purity for most uses. If a higher purity beryl lium oxide from an impure aqueous Sulfuric acid solution lium oxide is desired, then after separating the acidic containing dissolved beryllium sulfate by crystallizing mother liquor from the beryllium sulfate crystals and prior beryllium sulfate from the solution after concentrating to calcining, the separated beryllium sulfate crystals are it to about 50% to 55% by weight of sulfuric acid and 15 redissolved in an aqueous solution and treated such that, then calcining the crystallized beryllium Sulfate. upon recrystallization and mother liquor, separation, BeSO2HaO or BeSO-4HaO crystals are produced. Af ter this recrystallization and separation, the beryllium sul BACKGROUND OF THE INVENTION fate crystals would be subject to the calcining operation Several processes have been developed for the extrac to form high purity beryllium oxide. -

Beryllium, Inorganic and Soluble Salts
Beryllium, inorganic and soluble salts Evaluation of health hazards and proposal of a health based quality criterion for drinking water Environmental Project No. 1517, 2013 Title: Author: Beryllium, inorganic and soluble salts. Elsa Nielsen Evaluation of health hazards by exposure and Krestine Greve proposal of a health based quality criterion for Ole Ladefoged drinking water John Christian Larsen Division of Toxicology and Risk Assessment. National Food Institute, Technical University of Denmark Published by: The Danish Environmental Protection Agency Strandgade 29 1401 Copenhagen K Denmark www.mst.dk/english Year: ISBN no. Authored in 2009 978-87-93026-72-8 Published in 2013 Disclaimer: When the occasion arises, the Danish Environmental Protection Agency will publish reports and papers concerning research and development projects within the environmental sector, financed by study grants provided by the Danish Environmental Protection Agency. It should be noted that such publications do not necessarily reflect the position or opinion of the Danish Environmental Protection Agency. However, publication does indicate that, in the opinion of the Danish Environmental Protection Agency, the content represents an important contribution to the debate surrounding Danish environmental policy. Sources must be acknowledged. Content CONTENT 3 PREFACE 5 1 GENERAL DESCRIPTION 6 1.1 IDENTITY AND PHYSICAL / CHEMICAL PROPERTIES 6 1.2 PRODUCTION AND USE 6 1.3 ENVIRONMENTAL OCCURRENCE AND FATE 9 1.3.1 Air 9 1.3.2 Water 10 1.3.3 Soil 10 1.3.4 Foodstuffs 11 -

0805; R-0805-PL Recommended Use and to Be Used in Accordance with Manufacturer Instructions Or Under the Direct Guidance of the Restrictions Manufacturer
SAFETY DATA SHEET According to 29 CFR 1910.1200 Hazard Communication Standard 2012 (HazCom 2012) SECTION 1: Identification Product identifier Product name Fluoride Masking Agent Product number R-0805; R-0805-PL Recommended use and To be used in accordance with manufacturer instructions or under the direct guidance of the restrictions manufacturer. Manufacturer Taylor Technologies, Inc. 31 Loveton Circle Sparks, MD 21152 Phone: (410) 472-4340 Emergency phone: (800) 837-8548 SECTION 2: Hazard(s) identification Physical hazards Not applicable Health hazards Carcinogenicity Category 1A Sensitization, skin Category 1 Specific target organ toxicity, repeat exposure Category 1 Environmental hazards Not currently regulated by OSHA. For additional information, refer to section 12 of the SDS. Label elements Hazard pictograms Signal word Danger Hazard statements May cause cancer. May cause allergic skin reaction. Causes damage to organs through prolonged or repeated exposure. Precautionary statements Prevention Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Wear protective gloves/protective clothing/eye protection/face protection if contact is likely to occur. Contaminated work clothing must not be allowed out of the workplace. Do not breathe dust/fumes/gas/mists/vapors/spray. Wash skin thoroughly after handling. Do not eat, drink, or smoke when using this product. Response IF EXPOSED OR CONCERNED: Get medical advice/attention. IF ON SKIN: Wash with plenty of water. IF SKIN IRRITATION OR RASH OCCURS: Get medical advice/attention. Wash contaminated clothing before reuse. Get medical advice/attention if you feel unwell. Storage Store out of direct sunlight between 36°F−85°F. -

Beryllium and Beryllium Compounds
Report on Carcinogens, Fourteenth Edition For Table of Contents, see home page: http://ntp.niehs.nih.gov/go/roc Beryllium and Beryllium Compounds may result from binding of ionic beryllium to nucleic acids, which can cause infidelity of DNA replication (Leonard and Lauwerys 1987). CAS No. 7440-41-7 (Beryllium) Properties No separate CAS No. assigned for beryllium compounds as a class Beryllium is a silver-gray to grayish-white group II metallic element Known to be human carcinogens with an atomic weight of 9.01, melting point of 1,287°C, boiling point First listed in the Second Annual Report on Carcinogens (1981) of 2,970°C, and density of 1.85 at 20°C. It has a close-packed hexag- onal crystal structure and has several unique chemical properties. It Also known as Be is the lightest of all solid and chemically stable substances and has a Carcinogenicity very high melting point, specific heat, heat of fusion, and strength- to-weight ratio. Beryllium is lighter than aluminum, but it is over Beryllium and beryllium compounds are known to be human car 40% more rigid and approximately one-third more elastic than steel. cinogens based on sufficient evidence of carcinogenicity from stud- It is insoluble in water but soluble in acids and alkalis. It has excel- ies in humans. Beryllium and beryllium compounds were first listed lent electrical and thermal conductivity and is not magnetic. At ordi- in the Second Annual Report on Carcinogens as reasonably antici nary temperatures, beryllium resists oxidation in air; however, a thin pated to be human carcinogens based on sufficient evidence of car- film of beryllium oxide forms on the surface, making it highly resis- cinogenicity from studies in experimental animals.