Growth Hormone and Pituitary Adenomas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VIII. Hypothalamo Pituitary Axis Bone Turnover
Drugs affecting the hypothalamic – pituitary axis Regina Demlová, FÚ LF MU The hypothalamo-pituitary axis is the unit formed by the hypothalamus and pituitary gland , which exerts control over many parts of the endocrine system. the nervous system regulates the endocrine system and endocrine activity modulates the activity of the CNS. Hormones (peptides) – receptors – muscles, bones, fat tissue A/ membrane R → AC – catalyze the conversion of ATP to cAMP and pyrophosphate - a regulatory signal via specific cAMP-binding proteins, either transcription factors or other enzymes (e.g., cAMP- dependent kinases). B/ cytosolic receptor → translocation to the nucleus in a form capable of interacting with the DNA. Controlling the hypothalamic-pituitary-target organ axis : . important factor Negative feedback the action of hypothalamic hormones may be inhibited : A/ by long feedback patway from the target gland hormone or B/ by short feedback pathway from the pituitary hormone. There may also be direct feedback from the target gland hormone to the pituitary gland. Input is also received at the hypothalamus from higher brain centres Negative feedback CNS (sensoric, psychogenic) HYPOTHALAMIC HORMONES Releasing factors Long negative „feedback“ pathway Short negative „feedback“ pathway PITUITARY GLAND „trophs“ TSH ACTH FSH LH peripheral endocrine tissue gland Endocrinopathy • any disease due to disorder of the endocrine system A. the hypothalamic-pituitary-target organ axis • Peripheral - primary - arise at the level of peripheral endocrine.glands -

CS/HB 1041 Controlled Substances SPONSOR(S): Criminal Justice Subcommittee; Berman TIED BILLS: IDEN./SIM
HOUSE OF REPRESENTATIVES STAFF ANALYSIS BILL #: CS/HB 1041 Controlled Substances SPONSOR(S): Criminal Justice Subcommittee; Berman TIED BILLS: IDEN./SIM. BILLS: CS/SB 1448 REFERENCE ACTION ANALYST STAFF DIRECTOR or BUDGET/POLICY CHIEF 1) Criminal Justice Subcommittee 13 Y, 0 N, As Jones Cunningham CS 2) Justice Appropriations Subcommittee 3) Judiciary Committee SUMMARY ANALYSIS Chapter 893, F.S., sets forth the Florida Comprehensive Drug Abuse Prevention and Control Act and classifies controlled substances into five categories, known as schedules. These schedules are used to regulate the manufacture, distribution, preparation and dispensing of the substances listed therein. The distinguishing factors between the different drug schedules are the “potential for abuse” of the substance listed therein and whether there is a currently accepted medical use for the substance. Schedule III substances have a potential for abuse less than the substances contained in schedules I and II, and have an accepted medical use in treatment in the United States. Some examples of schedule III substances are barbituric acid, nalorphine, and anabolic steroids. The bill amends s. 893.03(3), F.S., to add the following substances to schedule III of Florida’s controlled substance schedules: hCG; CJC-1295; GHRH; GHRP-6; HGH; Somatropin; and Tesamorelin. As a result, the criminal penalties relating to the possession, sale, manufacture, delivery, etc. of schedule III controlled substances will apply to these newly added substances. The bill will likely have a negative fiscal impact on the Florida Department of Law Enforcement and the Department of Corrections. See fiscal section. The bill is effective on October 1, 2013. -

Restoring Youth
Restoring Youth The New Science of Human Growth Hormone Therapy Dr. Richard Gaines TABLE OF CONTENTS CHAPI'ER 1 Introduction to Human Growth 1 Hormone Replacement Therapy SECION 1 History of Growth Hormone Use 2 SECION 2 HGH and Hollywood 5 SECION 3 The Benefits of Growth Hormone 8 SECION 4 How HGH Levels are Tested 17 SECION 5 Using Growth Hormone 23 SECION 6 What are the Side Effects? 28 SECION 7 How Much does HGH Cost? 34 SECION 8 Buying Human Growth Hormone 38 SECION 9 Is HGH Legal? 44 CHAPI'ER 2 Related Hormones 47 SECION 1 Testosterone and HGH 48 SECION 2 Thyroid Hormone and HGH 53 SECION 3 Cortisol and Adrenal Fatigue 55 SECION 4 Insulin-like Growth Factor 57 SECION 5 GHRH and Sermorelin 60 SECION 6 Other Hormones and HGH 63 SECION 7 HGH Secretagogues 67 SECION 8 How to Boost HGH Naturally 74 CHAPI'ER 3 HGH Therapy for Men 79 SECION 1 Men and Comprehensive HRT 8o SECION 2 HGH Benefits for Men 86 SECION 3 HGH and Andropause 89 SECION 4 Adrenal Fatigue in Men 94 SECION 5 Men: Is HGH Right for You? 99 CHAPTER 4 HGH Therapy for Women 102 SECION 1 Women and Comprehensive HRT 103 SECION 2 HGH Benefits for Women 110 SECION 3 HGH and Menopause 114 SECION 4 Low Thyroid Syndrome in Women 122 SECION 5 Adrenal Fatigue in Women 128 SECION 6 Women and Bioidentical Hormones 131 SECION 7 Women: Is HGH Right for You? 136 SECION 8 One Woman's Story 139 CHAPTER 5 Choosing the Right HRT Provider 142 SECION 1 Things to Know 143 SECION 2 About HealthGAINS 150 SECION 3 FAQs Frequently Asked Questions 155 CHAPTER 6 Clinical Research 161 SECION 1 What Doctors Say 162 SECION 2 General Health 165 SECION 3 The Brain 168 SECION 4 Weight Loss 171 SECION 5 Anti-Aging 173 SECION 6 Article (Reprint) 175 Low T and Growth Hormone CHAPTER7 Product Information 179 SECION 1 FDA-Approved HGH 180 SECION 2 Illegal HGH 184 SECION 3 Related HGH Hormones 188 SECION 4 Rx HGH Peptides 193 SECION 5 HGH Oral Peptide Secretagogues 196 SECION 6 Homeopathic HGH 199 SECION 7 HGH Supplements 201 Preface THE CONTENT CONTAINED HEREIN IS MEANT TO BE INFORMATIVE ONLY. -
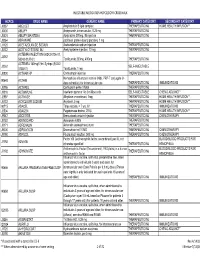
Injectable Medication Hcpcs/Dofr Crosswalk
INJECTABLE MEDICATION HCPCS/DOFR CROSSWALK HCPCS DRUG NAME GENERIC NAME PRIMARY CATEGORY SECONDARY CATEGORY J0287 ABELCET Amphotericin B lipid complex THERAPEUTIC INJ HOME HEALTH/INFUSION** J0400 ABILIFY Aripiprazole, intramuscular, 0.25 mg THERAPEUTIC INJ J0401 ABILIFY MAINTENA Apriprazole 300mg, IM injection THERAPEUTIC INJ J9264 ABRAXANE paclitaxel protein-bound particles, 1 mg J1120 ACETAZOLAMIDE SODIUM Acetazolamide sodium injection THERAPEUTIC INJ J0132 ACETYLCYSTEINE INJ Acetylcysteine injection, 10 mg THERAPEUTIC INJ ACTEMRA INJECTION (50242-0136-01, J3262 50242-0137-01) Tocilizumab 200mg, 400mg THERAPEUTIC INJ ACTEMRA 162mg/0.9ml Syringe (50242- J3262 SELF-INJECTABLE 0138-01) Tocilizumab, 1 mg J0800 ACTHAR HP Corticotropin injection THERAPEUTIC INJ Hemophilus influenza b vaccine (Hib), PRP-T conjugate (4- 90648 ACTHIB dose schedule), for intramuscular use THERAPEUTIC INJ IMMUNIZATIONS J0795 ACTHREL Corticorelin ovine triflutal THERAPEUTIC INJ J9216 ACTIMMUNE Interferon gamma 1-b 3 miillion units SELF-INJECTABLE CHEMO ADJUNCT* J2997 ACTIVASE Alteplase recombinant, 1mg THERAPEUTIC INJ HOME HEALTH/INFUSION** J0133 ACYCLOVIR SODIUM Acyclovir, 5 mg THERAPEUTIC INJ HOME HEALTH/INFUSION** 90715 ADACEL Tdap vaccine, > 7 yrs, IM THERAPEUTIC INJ IMMUNIZATIONS J2504 ADAGEN Pegademase bovine, 25 IU THERAPEUTIC INJ HOME HEALTH/INFUSION** J9042 ADCETRIS Brentuximab vedotin Injection THERAPEUTIC INJ CHEMOTHERAPY J0153 ADENOCARD Adenosine 6 MG THERAPEUTIC INJ J0171 ADRENALIN Adrenalin (epinephrine) inject THERAPEUTIC INJ J9000 ADRIAMYCIN Doxorubicin -

Ibm Micromedex® Carenotes Titles by Category
IBM MICROMEDEX® CARENOTES TITLES BY CATEGORY DECEMBER 2019 © Copyright IBM Corporation 2019 All company and product names mentioned are used for identification purposes only and may be trademarks of their respective owners. Table of Contents IBM Micromedex® CareNotes Titles by Category Allergy and Immunology ..................................................................................................................2 Ambulatory.......................................................................................................................................3 Bioterrorism ...................................................................................................................................18 Cardiology......................................................................................................................................18 Critical Care ...................................................................................................................................20 Dental Health .................................................................................................................................22 Dermatology ..................................................................................................................................23 Dietetics .........................................................................................................................................24 Endocrinology & Metabolic Disease ..............................................................................................26 -

Anterior Pituitary Hormones
HYPOTHALAMIC AND ANTERIOR PITUITARY HORMONES Dr. Nisha Department Of Pharmacology HYPOTHALAMIC-PITUITARY RELATIONSHIP HYPOTHALAMIC-PITUITARY RELATIONSHIP . Pituitary gland is connected to hypothalamus by the stalk that contains neurosecretory fibers, capillaries & the hypophyseal portal system that drains the hypothalamus & perfuses the anterior pituitary by a number of releasing factors (RF) or releasing hormones. These hypothalamic releasing factors/hormones stimulate the anterior pituitary to produce & secrete a number of tropic hormones, that in turn stimulate target glands to secrete hormones which finally act on the cells of target organs far away from the site of their release. Pituitary hormone secretions are regulated by negative feedback mechanisms. These pathways are “long” as well as “short” negative feedback pathways. If the hormone secreted affects both the hypothalamus & pituitary, then it makes a long negative feedback loop. If they affect only pituitary, then it is a short negative feedback loop. PITUITARY HORMONES (Overview) GROWTH HORMONE (GH) . GH secretion is high in newborn till 4 yrs of age & starts declining after 25 yrs. Pharmacological Effects of GH: -↑protein synthesis (anabolic) - Positive N2 balance (↑AA uptake in cells) - Anabolic effects are mediated by somatomedins (IGF-1 & IGF-2) - Initially insulin like effects & later anti-insulin effects: ↑blood glucose, ↑FFA mobilization, ketone body formation. - IGF-1 inhibit GH release from ant. pituitary & stimulate GHRIH release from hypothalamus. GH Regulating Factors . GHRH (Growth hormone releasing hormone):- released from hypothalamus, regulate GH release. GHRH analogue: Sermorelin- used as diagnostic agent for testing pituitary GH secretion capability in childhood short stature. GHRIH (Growth hormone release-inhibiting hormone) – Somatostatin It inhibits secretion of GH, TSH, insulin & gastrin. -

INN Working Document 05.179 Update December 2010
INN Working Document 05.179 Update December 2010 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) INN Working Document 05.179 Distr.: GENERAL ENGLISH ONLY 12/2010 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Essential Medicines and Pharmaceutical Policies (EMP) International Nonproprietary Names (INN) for biological and biotechnological substances (a review) © World Health Organization 2010 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

International Nonproprietary Names (Inn) for Biological and Biotechnological Substances
INN Working Document 05.179 Distr.: GENERAL ENGLISH ONLY 15/06/2006 INTERNATIONAL NONPROPRIETARY NAMES (INN) FOR BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES (A REVIEW) Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines (QSM) Medicines Policy and Standards (PSM) Department CONTENTS 0. INTRODUCTION…………………………………….........................................................................................v 1. PHARMACOLOGICAL CLASSIFICATION OF BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES……………………………………................................1 2. CURRENT STATUS OF EXISTING STEMS OR SYSTEMS FOR BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES…………………….3 2.1 Groups with respective stems ……………………………………………………………………3 2.2 Groups with respective pre-stems………………………………………………………………4 2.3 Groups with INN schemes………………………………………………………………………….4 2.4 Groups without respective stems / pre-stems and without INN schemes…..4 3. GENERAL POLICIES FOR BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES……………………………………………………………………………………………………...5 3.1 General policies for blood products……………………………………………………………5 3.2 General policies for fusion proteins……………………………………………………………5 3.3 General policies for gene therapy products………………………………………………..5 3.4 General policies for glycosylated and non-glycosylated compounds………...6 3.5 General policies for immunoglobulins……………………………………………………….7 3.6 General polices for monoclonal antibodies………………………………………………..7 3.7 General polices for skin substitutes……………………………………………………………9 3.8 General policies for transgenic products……………………………………………………9 -

(INN) for Biological and Biotechnological Substances
WHO/EMP/RHT/TSN/2019.1 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) 2019 WHO/EMP/RHT/TSN/2019.1 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) 2019 International Nonproprietary Names (INN) Programme Technologies Standards and Norms (TSN) Regulation of Medicines and other Health Technologies (RHT) Essential Medicines and Health Products (EMP) International Nonproprietary Names (INN) for biological and biotechnological substances (a review) FORMER DOCUMENT NUMBER: INN Working Document 05.179 © World Health Organization 2019 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications –whether for sale or for non-commercial distribution– should be addressed to WHO Press through the WHO website (www.who.int/about/licensing/copyright_form/en/index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. -

2019 Table of Drugs
2019 Table of Drugs Questions regarding coding and billing guidance should be submitted to the insurer in whose jurisdiction a claim would be filed. For private sector health insurance systems, please contact the individual private insurance entity. For Medicaid systems, please contact the Medicaid Agency in the state in which the claim is being filed. For Medicare, contact the Medicare contractor. IA - Intra-arterial administration IV - Intravenous administration IM - Intramuscular administration IT - Intrathecal SC - Subcutaneous administration INH - Administration by inhaled solution VAR - Various routes of administration OTH - Other routes of administration ORAL - Administered orally Intravenous administration includes all methods, such as gravity infusion, injections, and timed pushes. The ‘VAR’ posting denotes various routes of administration and is used for drugs that are commonly administered into joints, cavities, tissues, or topical applications, in addition to other parenteral administrations. Listings posted with ‘OTH’ indicate other administration methods, such as suppositories or catheter injections. A Abatacept 10 mg IV J0129 Abbokinase, see Urokinase Abbokinase, Open Cath, see Urokinase Abciximab 10 mg IV J0130 Abelcet, see Amphotericin B Lipid Complex ABLC, see Amphotericin B AbobotulinumtoxintypeA 5 units IM J0586 Acetaminophen 10 mg IV J0131 Acetazolamide sodium up to 500 mg IM, IV J1120 Acetylcysteine, IVection 100 mg IV J0132 Acetylcysteine, unit dose form per gram INH J7604, J7608 1 Achromycin, see Tetracycline Actemra, -
(12) Patent Application Publication (10) Pub. No.: US 2002/0010208A1 Shashoua Et Al
US 2002001 0208A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0010208A1 Shashoua et al. (43) Pub. Date: Jan. 24, 2002 (54) DHA-PHARMACEUTICAL AGENT Related U.S. Application Data CONJUGATES OF TAXANES (63) Continuation of application No. 09/135,291, filed on (76) Inventors: Victor Shashoua, Brookline, MA (US); Aug. 17, 1998, now abandoned, which is a continu Charles Swindell, Merion, PA (US); ation of application No. 08/651,312, filed on May 22, Nigel Webb, Bryn Mawr, PA (US); 1996, now Pat. No. 5,795,909. Matthews Bradley, Layton, PA (US) Publication Classification Correspondence Address: Edward R. Gates, Esq. (51) Int. Cl." ............................................ A61K 31/337 Wolf, Greenfield & Sacks, P.C. (52) U.S. Cl. .............................................................. 514/449 600 Atlantic Avenue Boston, MA 02210 (US) (57) ABSTRACT The invention provides conjugates of cis-docosahexaenoic (21) Appl. No.: 09/846,838 acid and pharmaceutical agents useful in treating noncentral nervous System conditions. Methods for Selectively target 22) Filled: Mayy 1, 2001 ingg pharmaceuticalp agents9. to desired tissues are pprovided. Patent Application Publication Jan. 24, 2002 Sheet 1 of 14 US 2002/0010208A1 1 OO 5 O -5OO - 1 OO-9 -8 -7 -6 -5 -4 LOG-10 OF SAMPLE CONCENTRATION (MOLAR) CCRF-CEM-o- SR ----- RPM-8226----- K-562- - -A - - HL-60 (TB) -g- - MOLT4: ... O Fig. 1 1 OO 5 O -5OO -1 O O -8 -7 -6 -5 -4 -- 9 LOGo OF SAMPLE CONCENTRATION (MOLAR) A549/ATCC-o-NS326. NCEKVX 28. --Q-- NCI-H322M-...-a---Eidsf8::... NC-H522--O-- HOP-62---Fig. 2 a-- NC-H460.-------- Patent Application Publication Jan. -
International Nonproprietary Names (INN) for Biological and Biotechnological Substances
WHO/EMP/RHT/TSN/2014.1 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) WHO/EMP/RHT/TSN/2014.1 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) International Nonproprietary Names (INN) Programme Technologies Standards and Norms (TSN) Regulation of Medicines and other Health Technologies (RHT) Essential Medicines and Health Products (EMP) International Nonproprietary Names (INN) for biological and biotechnological substances (a review) FORMER DOCUMENT NUMBER: INN Working Document 05.179 © World Health Organization 2014 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int ) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected] ). Requests for permission to reproduce or translate WHO publications –whether for sale or for non- commercial distribution– should be addressed to WHO Press through the WHO website (www.who.int/about/licensing/copyright_form/en/index.html ). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned.