INN Working Document 05.179 Distr.: GENERAL ENGLISH ONLY 08/11/2009
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix
United States International Trade Commission Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix USITC Publication 4208 December 2010 U.S. International Trade Commission COMMISSIONERS Deanna Tanner Okun, Chairman Irving A. Williamson, Vice Chairman Charlotte R. Lane Daniel R. Pearson Shara L. Aranoff Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix Publication 4208 December 2010 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 15, 2010, set forth at the end of this publication, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the United States International Trade Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement changes to the Pharmaceutical Appendix, effective on January 1, 2011. Table 1 International Nonproprietary Name (INN) products proposed for addition to the Pharmaceutical Appendix to the Harmonized Tariff Schedule INN CAS Number Abagovomab 792921-10-9 Aclidinium Bromide 320345-99-1 Aderbasib 791828-58-5 Adipiplon 840486-93-3 Adoprazine 222551-17-9 Afimoxifene 68392-35-8 Aflibercept 862111-32-8 Agatolimod -

VIII. Hypothalamo Pituitary Axis Bone Turnover
Drugs affecting the hypothalamic – pituitary axis Regina Demlová, FÚ LF MU The hypothalamo-pituitary axis is the unit formed by the hypothalamus and pituitary gland , which exerts control over many parts of the endocrine system. the nervous system regulates the endocrine system and endocrine activity modulates the activity of the CNS. Hormones (peptides) – receptors – muscles, bones, fat tissue A/ membrane R → AC – catalyze the conversion of ATP to cAMP and pyrophosphate - a regulatory signal via specific cAMP-binding proteins, either transcription factors or other enzymes (e.g., cAMP- dependent kinases). B/ cytosolic receptor → translocation to the nucleus in a form capable of interacting with the DNA. Controlling the hypothalamic-pituitary-target organ axis : . important factor Negative feedback the action of hypothalamic hormones may be inhibited : A/ by long feedback patway from the target gland hormone or B/ by short feedback pathway from the pituitary hormone. There may also be direct feedback from the target gland hormone to the pituitary gland. Input is also received at the hypothalamus from higher brain centres Negative feedback CNS (sensoric, psychogenic) HYPOTHALAMIC HORMONES Releasing factors Long negative „feedback“ pathway Short negative „feedback“ pathway PITUITARY GLAND „trophs“ TSH ACTH FSH LH peripheral endocrine tissue gland Endocrinopathy • any disease due to disorder of the endocrine system A. the hypothalamic-pituitary-target organ axis • Peripheral - primary - arise at the level of peripheral endocrine.glands -

CS/HB 1041 Controlled Substances SPONSOR(S): Criminal Justice Subcommittee; Berman TIED BILLS: IDEN./SIM
HOUSE OF REPRESENTATIVES STAFF ANALYSIS BILL #: CS/HB 1041 Controlled Substances SPONSOR(S): Criminal Justice Subcommittee; Berman TIED BILLS: IDEN./SIM. BILLS: CS/SB 1448 REFERENCE ACTION ANALYST STAFF DIRECTOR or BUDGET/POLICY CHIEF 1) Criminal Justice Subcommittee 13 Y, 0 N, As Jones Cunningham CS 2) Justice Appropriations Subcommittee 3) Judiciary Committee SUMMARY ANALYSIS Chapter 893, F.S., sets forth the Florida Comprehensive Drug Abuse Prevention and Control Act and classifies controlled substances into five categories, known as schedules. These schedules are used to regulate the manufacture, distribution, preparation and dispensing of the substances listed therein. The distinguishing factors between the different drug schedules are the “potential for abuse” of the substance listed therein and whether there is a currently accepted medical use for the substance. Schedule III substances have a potential for abuse less than the substances contained in schedules I and II, and have an accepted medical use in treatment in the United States. Some examples of schedule III substances are barbituric acid, nalorphine, and anabolic steroids. The bill amends s. 893.03(3), F.S., to add the following substances to schedule III of Florida’s controlled substance schedules: hCG; CJC-1295; GHRH; GHRP-6; HGH; Somatropin; and Tesamorelin. As a result, the criminal penalties relating to the possession, sale, manufacture, delivery, etc. of schedule III controlled substances will apply to these newly added substances. The bill will likely have a negative fiscal impact on the Florida Department of Law Enforcement and the Department of Corrections. See fiscal section. The bill is effective on October 1, 2013. -

Primary Prophylaxis for Venous Thromboembolism in Ambulatory Cancer Patients Receiving Chemotherapy (Review) I Copyright © 2016 the Cochrane Collaboration
Cochrane Database of Systematic Reviews Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy (Review) Di Nisio M, Porreca E, Candeloro M, De Tursi M, Russi I, Rutjes AWS | downloaded: 25.9.2021 Di Nisio M, Porreca E, Candeloro M, De Tursi M, Russi I, Rutjes AWS. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No.: CD008500. DOI: 10.1002/14651858.CD008500.pub4. www.cochranelibrary.com https://doi.org/10.7892/boris.92495 Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy (Review) Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. source: TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 SUMMARY OF FINDINGS FOR THE MAIN COMPARISON . ..... 4 BACKGROUND .................................... 7 OBJECTIVES ..................................... 7 METHODS ...................................... 8 RESULTS....................................... 10 Figure1. ..................................... 11 Figure2. ..................................... 15 Figure3. ..................................... 18 Figure4. ..................................... 19 Figure5. ..................................... 20 Figure6. ..................................... 21 ADDITIONALSUMMARYOFFINDINGS . 23 DISCUSSION .................................... -

WHO Drug Information Vol 23, No
WHO Drug Information Vol 23, No. 1, 2009 World Health Organization WHO Drug Information Contents Quality of Medicines Erlotinib: Use in hepatic impairment and advanced solid tumours 32 WHO medicines prequalification: Generic piperacillin and tazobactam: progress in 2008 3 medication errors 33 Safety of artemisinin combination therapy: Herbal and Traditional pregnant women 33 Medicines WHO Congress on Traditional Medicine Regulatory Action and News and the Beijing Declaration 8 Efalizumab: suspension of marketing authorization 35 Contusugene ladenovec: withdrawal of Biomedicines Update marketing application 36 Global norms and standards for biological Paliperidone: withdrawal of application quality, safety and efficacy 12 for extension of indication 36 First EMEA meeting of Committee for Safety of Medicines Advanced Therapies 36 The mobile laboratory: a new concept in medicines surveillance 16 Consultation document International Pharmacopoeia Pharmacovigilance Focus Oxytocin 38 Oxytocin injection 43 Monitoring the safety of off-label medicine use 21 Drotrecogin alfa: what relation to Recent Publications, thrombosis? 22 Information and Events Low availability, high prices keep Safety and Efficacy Issues essential medicines out of reach 46 Genetic basis of adverse drug events 26 Sources and prices of selected Anaesthetic infusion with pain pumps: medicines for children 46 articular chondrolysis 26 Two international summer courses Atomoxetine: serious liver injury 27 in the Netherlands 47 Drotrecogin alfa: ongoing safety review 28 Global politics of pharmaceutical Clopidogrel bisulfate: ongoing safety monopoly 48 review 29 Modafinil: adverse skin and psychiatric reactions 30 Recommended International Levothyroxine update 30 Nonproprietary Names: Temsirolimus: hypersensitivity reactions 31 List 61 Continued vaccination with Gardasil® 32 49 1 World Health Organization WHO Drug Information Vol 23, No. -

Primary Prophylaxis for Venous Thromboembolism in Ambulatory Cancer Patients Receiving Chemotherapy (Review)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Bern Open Repository and Information System (BORIS) Cochrane Database of Systematic Reviews Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy (Review) Di Nisio M, Porreca E, Candeloro M, De Tursi M, Russi I, Rutjes AWS Di Nisio M, Porreca E, Candeloro M, De Tursi M, Russi I, Rutjes AWS. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No.: CD008500. DOI: 10.1002/14651858.CD008500.pub4. www.cochranelibrary.com Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy (Review) Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 SUMMARY OF FINDINGS FOR THE MAIN COMPARISON . ..... 4 BACKGROUND .................................... 7 OBJECTIVES ..................................... 7 METHODS ...................................... 8 RESULTS....................................... 10 Figure1. ..................................... 11 Figure2. ..................................... 15 Figure3. ..................................... 18 Figure4. ..................................... 19 Figure5. ..................................... 20 Figure6. ..................................... 21 ADDITIONALSUMMARYOFFINDINGS -

Restoring Youth
Restoring Youth The New Science of Human Growth Hormone Therapy Dr. Richard Gaines TABLE OF CONTENTS CHAPI'ER 1 Introduction to Human Growth 1 Hormone Replacement Therapy SECION 1 History of Growth Hormone Use 2 SECION 2 HGH and Hollywood 5 SECION 3 The Benefits of Growth Hormone 8 SECION 4 How HGH Levels are Tested 17 SECION 5 Using Growth Hormone 23 SECION 6 What are the Side Effects? 28 SECION 7 How Much does HGH Cost? 34 SECION 8 Buying Human Growth Hormone 38 SECION 9 Is HGH Legal? 44 CHAPI'ER 2 Related Hormones 47 SECION 1 Testosterone and HGH 48 SECION 2 Thyroid Hormone and HGH 53 SECION 3 Cortisol and Adrenal Fatigue 55 SECION 4 Insulin-like Growth Factor 57 SECION 5 GHRH and Sermorelin 60 SECION 6 Other Hormones and HGH 63 SECION 7 HGH Secretagogues 67 SECION 8 How to Boost HGH Naturally 74 CHAPI'ER 3 HGH Therapy for Men 79 SECION 1 Men and Comprehensive HRT 8o SECION 2 HGH Benefits for Men 86 SECION 3 HGH and Andropause 89 SECION 4 Adrenal Fatigue in Men 94 SECION 5 Men: Is HGH Right for You? 99 CHAPTER 4 HGH Therapy for Women 102 SECION 1 Women and Comprehensive HRT 103 SECION 2 HGH Benefits for Women 110 SECION 3 HGH and Menopause 114 SECION 4 Low Thyroid Syndrome in Women 122 SECION 5 Adrenal Fatigue in Women 128 SECION 6 Women and Bioidentical Hormones 131 SECION 7 Women: Is HGH Right for You? 136 SECION 8 One Woman's Story 139 CHAPTER 5 Choosing the Right HRT Provider 142 SECION 1 Things to Know 143 SECION 2 About HealthGAINS 150 SECION 3 FAQs Frequently Asked Questions 155 CHAPTER 6 Clinical Research 161 SECION 1 What Doctors Say 162 SECION 2 General Health 165 SECION 3 The Brain 168 SECION 4 Weight Loss 171 SECION 5 Anti-Aging 173 SECION 6 Article (Reprint) 175 Low T and Growth Hormone CHAPTER7 Product Information 179 SECION 1 FDA-Approved HGH 180 SECION 2 Illegal HGH 184 SECION 3 Related HGH Hormones 188 SECION 4 Rx HGH Peptides 193 SECION 5 HGH Oral Peptide Secretagogues 196 SECION 6 Homeopathic HGH 199 SECION 7 HGH Supplements 201 Preface THE CONTENT CONTAINED HEREIN IS MEANT TO BE INFORMATIVE ONLY. -
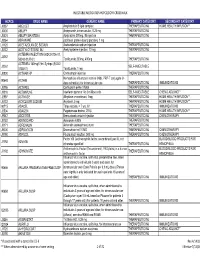
Injectable Medication Hcpcs/Dofr Crosswalk
INJECTABLE MEDICATION HCPCS/DOFR CROSSWALK HCPCS DRUG NAME GENERIC NAME PRIMARY CATEGORY SECONDARY CATEGORY J0287 ABELCET Amphotericin B lipid complex THERAPEUTIC INJ HOME HEALTH/INFUSION** J0400 ABILIFY Aripiprazole, intramuscular, 0.25 mg THERAPEUTIC INJ J0401 ABILIFY MAINTENA Apriprazole 300mg, IM injection THERAPEUTIC INJ J9264 ABRAXANE paclitaxel protein-bound particles, 1 mg J1120 ACETAZOLAMIDE SODIUM Acetazolamide sodium injection THERAPEUTIC INJ J0132 ACETYLCYSTEINE INJ Acetylcysteine injection, 10 mg THERAPEUTIC INJ ACTEMRA INJECTION (50242-0136-01, J3262 50242-0137-01) Tocilizumab 200mg, 400mg THERAPEUTIC INJ ACTEMRA 162mg/0.9ml Syringe (50242- J3262 SELF-INJECTABLE 0138-01) Tocilizumab, 1 mg J0800 ACTHAR HP Corticotropin injection THERAPEUTIC INJ Hemophilus influenza b vaccine (Hib), PRP-T conjugate (4- 90648 ACTHIB dose schedule), for intramuscular use THERAPEUTIC INJ IMMUNIZATIONS J0795 ACTHREL Corticorelin ovine triflutal THERAPEUTIC INJ J9216 ACTIMMUNE Interferon gamma 1-b 3 miillion units SELF-INJECTABLE CHEMO ADJUNCT* J2997 ACTIVASE Alteplase recombinant, 1mg THERAPEUTIC INJ HOME HEALTH/INFUSION** J0133 ACYCLOVIR SODIUM Acyclovir, 5 mg THERAPEUTIC INJ HOME HEALTH/INFUSION** 90715 ADACEL Tdap vaccine, > 7 yrs, IM THERAPEUTIC INJ IMMUNIZATIONS J2504 ADAGEN Pegademase bovine, 25 IU THERAPEUTIC INJ HOME HEALTH/INFUSION** J9042 ADCETRIS Brentuximab vedotin Injection THERAPEUTIC INJ CHEMOTHERAPY J0153 ADENOCARD Adenosine 6 MG THERAPEUTIC INJ J0171 ADRENALIN Adrenalin (epinephrine) inject THERAPEUTIC INJ J9000 ADRIAMYCIN Doxorubicin -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

A Abacavir Abacavirum Abakaviiri Abagovomab Abagovomabum
A abacavir abacavirum abakaviiri abagovomab abagovomabum abagovomabi abamectin abamectinum abamektiini abametapir abametapirum abametapiiri abanoquil abanoquilum abanokiili abaperidone abaperidonum abaperidoni abarelix abarelixum abareliksi abatacept abataceptum abatasepti abciximab abciximabum absiksimabi abecarnil abecarnilum abekarniili abediterol abediterolum abediteroli abetimus abetimusum abetimuusi abexinostat abexinostatum abeksinostaatti abicipar pegol abiciparum pegolum abisipaaripegoli abiraterone abirateronum abirateroni abitesartan abitesartanum abitesartaani ablukast ablukastum ablukasti abrilumab abrilumabum abrilumabi abrineurin abrineurinum abrineuriini abunidazol abunidazolum abunidatsoli acadesine acadesinum akadesiini acamprosate acamprosatum akamprosaatti acarbose acarbosum akarboosi acebrochol acebrocholum asebrokoli aceburic acid acidum aceburicum asebuurihappo acebutolol acebutololum asebutololi acecainide acecainidum asekainidi acecarbromal acecarbromalum asekarbromaali aceclidine aceclidinum aseklidiini aceclofenac aceclofenacum aseklofenaakki acedapsone acedapsonum asedapsoni acediasulfone sodium acediasulfonum natricum asediasulfoninatrium acefluranol acefluranolum asefluranoli acefurtiamine acefurtiaminum asefurtiamiini acefylline clofibrol acefyllinum clofibrolum asefylliiniklofibroli acefylline piperazine acefyllinum piperazinum asefylliinipiperatsiini aceglatone aceglatonum aseglatoni aceglutamide aceglutamidum aseglutamidi acemannan acemannanum asemannaani acemetacin acemetacinum asemetasiini aceneuramic -

Ibm Micromedex® Carenotes Titles by Category
IBM MICROMEDEX® CARENOTES TITLES BY CATEGORY DECEMBER 2019 © Copyright IBM Corporation 2019 All company and product names mentioned are used for identification purposes only and may be trademarks of their respective owners. Table of Contents IBM Micromedex® CareNotes Titles by Category Allergy and Immunology ..................................................................................................................2 Ambulatory.......................................................................................................................................3 Bioterrorism ...................................................................................................................................18 Cardiology......................................................................................................................................18 Critical Care ...................................................................................................................................20 Dental Health .................................................................................................................................22 Dermatology ..................................................................................................................................23 Dietetics .........................................................................................................................................24 Endocrinology & Metabolic Disease ..............................................................................................26 -

Anterior Pituitary Hormones
HYPOTHALAMIC AND ANTERIOR PITUITARY HORMONES Dr. Nisha Department Of Pharmacology HYPOTHALAMIC-PITUITARY RELATIONSHIP HYPOTHALAMIC-PITUITARY RELATIONSHIP . Pituitary gland is connected to hypothalamus by the stalk that contains neurosecretory fibers, capillaries & the hypophyseal portal system that drains the hypothalamus & perfuses the anterior pituitary by a number of releasing factors (RF) or releasing hormones. These hypothalamic releasing factors/hormones stimulate the anterior pituitary to produce & secrete a number of tropic hormones, that in turn stimulate target glands to secrete hormones which finally act on the cells of target organs far away from the site of their release. Pituitary hormone secretions are regulated by negative feedback mechanisms. These pathways are “long” as well as “short” negative feedback pathways. If the hormone secreted affects both the hypothalamus & pituitary, then it makes a long negative feedback loop. If they affect only pituitary, then it is a short negative feedback loop. PITUITARY HORMONES (Overview) GROWTH HORMONE (GH) . GH secretion is high in newborn till 4 yrs of age & starts declining after 25 yrs. Pharmacological Effects of GH: -↑protein synthesis (anabolic) - Positive N2 balance (↑AA uptake in cells) - Anabolic effects are mediated by somatomedins (IGF-1 & IGF-2) - Initially insulin like effects & later anti-insulin effects: ↑blood glucose, ↑FFA mobilization, ketone body formation. - IGF-1 inhibit GH release from ant. pituitary & stimulate GHRIH release from hypothalamus. GH Regulating Factors . GHRH (Growth hormone releasing hormone):- released from hypothalamus, regulate GH release. GHRH analogue: Sermorelin- used as diagnostic agent for testing pituitary GH secretion capability in childhood short stature. GHRIH (Growth hormone release-inhibiting hormone) – Somatostatin It inhibits secretion of GH, TSH, insulin & gastrin.